Microsecond-scale timing precision in rodent trigeminal primary afferents
- PMID: 25878266
- PMCID: PMC4397594
- DOI: 10.1523/JNEUROSCI.3876-14.2015
Microsecond-scale timing precision in rodent trigeminal primary afferents
Abstract
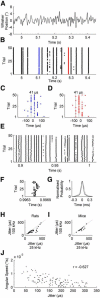
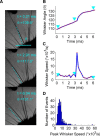
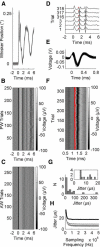
Communication in the nervous system occurs by spikes: the timing precision with which spikes are fired is a fundamental limit on neural information processing. In sensory systems, spike-timing precision is constrained by first-order neurons. We found that spike-timing precision of trigeminal primary afferents in rats and mice is limited both by stimulus speed and by electrophysiological sampling rate. High-speed video of behaving mice revealed whisker velocities of at least 17,000°/s, so we delivered an ultrafast "ping" (>50,000°/s) to single whiskers and sampled primary afferent activity at 500 kHz. Median spike jitter was 17.4 μs; 29% of neurons had spike jitter < 10 μs. These results indicate that the input stage of the trigeminal pathway has extraordinary spike-timing precision and very high potential information capacity. This timing precision ranks among the highest in biology.
Keywords: neural coding; trigeminal ganglion; vibrissa; whisker.
Copyright © 2015 the authors 0270-6474/15/355935-06$15.00/0.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
