In vivo PET imaging of the α4β2 nicotinic acetylcholine receptor as a marker for brain inflammation after cerebral ischemia
- PMID: 25878273
- PMCID: PMC6605174
- DOI: 10.1523/JNEUROSCI.3670-14.2015
In vivo PET imaging of the α4β2 nicotinic acetylcholine receptor as a marker for brain inflammation after cerebral ischemia
Abstract
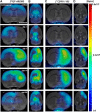
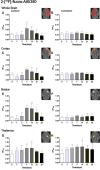
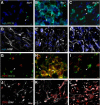
PET imaging of nicotinic acetylcholine receptors (nAChRs) could become an effective tool for the diagnosis and therapy evaluation of neurologic diseases. Despite this, the role of nAChRs α4β2 receptors after brain diseases such as cerebral ischemia and its involvement in inflammatory reaction is still largely unknown. To investigate this, we performed in parallel in vivo magnetic resonance imaging (MRI) and positron emission tomography (PET) with 2[(18)F]-fluoro-A85380 and [(11)C]PK11195 at 1, 3, 7, 14, 21, and 28 d after middle cerebral artery occlusion (MCAO) in rats. In the ischemic territory, PET with 2[(18)F]-fluoro-A85380 and [(11)C]PK11195 showed a progressive binding increase from days 3-7, followed by a progressive decrease from days 14-28 after cerebral ischemia onset. Ex vivo immunohistochemistry for the nicotinic α4β2 receptor and the mitochondrial translocator protein (18 kDa) (TSPO) confirmed the PET findings and demonstrated the overexpression of α4β2 receptors in both microglia/macrophages and astrocytes from days 7-28 after experimental ischemic stroke. Likewise, the role played by α4β2 receptors on neuroinflammation was supported by the increase of [(11)C]PK11195 binding in ischemic rats treated with the α4β2 antagonist dihydro-β-erythroidine hydrobromide (DHBE) at day 7 after MCAO. Finally, both functional and behavioral testing showed major impaired outcome at day 1 after ischemia onset, followed by a recovery of the sensorimotor function and dexterity from days 21-28 after experimental stroke. Together, these results suggest that the nicotinic α4β2 receptor could have a key role in the inflammatory reaction underlying cerebral ischemia in rats.
Keywords: 2[18F]-fluoro-A85380; PET; TSPO; [11C]PK11195; cerebral ischemia; α4β2.
Copyright © 2015 the authors 0270-6474/15/355998-12$15.00/0.
Figures




Similar articles
-
In vivo imaging of Α7 nicotinic receptors as a novel method to monitor neuroinflammation after cerebral ischemia.Glia. 2018 Aug;66(8):1611-1624. doi: 10.1002/glia.23326. Epub 2018 Mar 12. Glia. 2018. PMID: 29528142
-
[18F]DPA-714: direct comparison with [11C]PK11195 in a model of cerebral ischemia in rats.PLoS One. 2013;8(2):e56441. doi: 10.1371/journal.pone.0056441. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418569 Free PMC article.
-
Effects of minocycline on endogenous neural stem cells after experimental stroke.Neuroscience. 2012 Jul 26;215:174-83. doi: 10.1016/j.neuroscience.2012.04.036. Epub 2012 Apr 24. Neuroscience. 2012. PMID: 22542871
-
Radioligand imaging of α4β2* nicotinic acetylcholine receptors in Alzheimer's disease and Parkinson's disease.Q J Nucl Med Mol Imaging. 2014 Dec;58(4):376-86. Epub 2014 Nov 11. Q J Nucl Med Mol Imaging. 2014. PMID: 25387119 Review.
-
Positron emission tomography to image cerebral neuroinflammation in ischaemic stroke: a pilot study.Southampton (UK): NIHR Journals Library; 2020 Feb. Southampton (UK): NIHR Journals Library; 2020 Feb. PMID: 32023020 Free Books & Documents. Review.
Cited by
-
Role of Microglia in Stroke.Adv Neurobiol. 2024;37:405-422. doi: 10.1007/978-3-031-55529-9_23. Adv Neurobiol. 2024. PMID: 39207705 Review.
-
Disruption of normal brain distribution of [18F]Nifene to α4β2* nicotinic acetylcholinergic receptors in old B6129SF2/J mice and transgenic 3xTg-AD mice model of Alzheimer's disease: In Vivo PET/CT imaging studies.Neuroimage. 2025 Apr 1;309:121092. doi: 10.1016/j.neuroimage.2025.121092. Epub 2025 Feb 18. Neuroimage. 2025. PMID: 39978704 Free PMC article.
-
PET Imaging of Crossed Cerebellar Diaschisis after Long-Term Cerebral Ischemia in Rats.Contrast Media Mol Imaging. 2018 Dec 2;2018:2483078. doi: 10.1155/2018/2483078. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 30627057 Free PMC article.
-
TSPO Expression Modulatory Effect of Acetylcholinesterase Inhibitor in the Ischemic Stroke Rat Model.Cells. 2021 May 29;10(6):1350. doi: 10.3390/cells10061350. Cells. 2021. PMID: 34072449 Free PMC article.
-
Assessing the Effects of Cytoprotectants on Selective Neuronal Loss, Sensorimotor Deficit and Microglial Activation after Temporary Middle Cerebral Occlusion.Brain Sci. 2019 Oct 22;9(10):287. doi: 10.3390/brainsci9100287. Brain Sci. 2019. PMID: 31652564 Free PMC article.
References
-
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123:2321–2337. doi: 10.1093/brain/123.11.2321. - DOI - PubMed
-
- Bottlaender M, Valette H, Roumenov D, Dollé F, Coulon C, Ottaviani M, Hinnen F, Ricard M. Biodistribution and radiation dosimetry of 18F-fluoro-A-85380 in healthy volunteers. J Nucl Med. 2003;44:596–601. - PubMed
-
- Burghaus L, Schütz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schröder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res. 2000;76:385–388. doi: 10.1016/S0169-328X(00)00031-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
