Non-aggregating tau phosphorylation by cyclin-dependent kinase 5 contributes to motor neuron degeneration in spinal muscular atrophy
- PMID: 25878277
- PMCID: PMC4397602
- DOI: 10.1523/JNEUROSCI.3716-14.2015
Non-aggregating tau phosphorylation by cyclin-dependent kinase 5 contributes to motor neuron degeneration in spinal muscular atrophy
Abstract
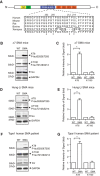
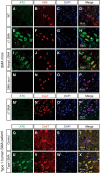
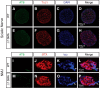
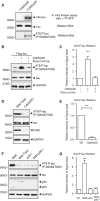
Mechanisms underlying motor neuron degeneration in spinal muscular atrophy (SMA), the leading inherited cause of infant mortality, remain largely unknown. Many studies have established the importance of hyperphosphorylation of the microtubule-associated protein tau in various neurodegenerative disorders, including Alzheimer's and Parkinson's diseases. However, tau phosphorylation in SMA pathogenesis has yet to be investigated. Here we show that tau phosphorylation on serine 202 (S202) and threonine 205 (T205) is increased significantly in SMA motor neurons using two SMA mouse models and human SMA patient spinal cord samples. Interestingly, phosphorylated tau does not form aggregates in motor neurons or neuromuscular junctions (NMJs), even at late stages of SMA disease, distinguishing it from other tauopathies. Hyperphosphorylation of tau on S202 and T205 is mediated by cyclin-dependent kinase 5 (Cdk5) in SMA disease condition, because tau phosphorylation at these sites is significantly reduced in Cdk5 knock-out mice; genetic knock-out of Cdk5 activating subunit p35 in an SMA mouse model also leads to reduced tau phosphorylation on S202 and T205 in the SMA;p35(-/-) compound mutant mice. In addition, expression of the phosphorylation-deficient tauS202A,T205A mutant alleviates motor neuron defects in a zebrafish SMA model in vivo and mouse motor neuron degeneration in culture, whereas expression of phosphorylation-mimetic tauS202E,T205E promotes motor neuron defects. More importantly, genetic knock-out of tau in SMA mice rescues synapse stripping on motor neurons, NMJ denervation, and motor neuron degeneration in vivo. Altogether, our findings suggest a novel mechanism for SMA pathogenesis in which hyperphosphorylation of non-aggregating tau by Cdk5 contributes to motor neuron degeneration.
Keywords: Cdk5; SMA; motor neuron; neurodegeneration; tau.
Copyright © 2015 the authors 0270-6474/15/356038-13$15.00/0.
Figures

Similar articles
-
Mitigating aberrant Cdk5 activation alleviates mitochondrial defects and motor neuron disease symptoms in spinal muscular atrophy.Proc Natl Acad Sci U S A. 2023 Nov 21;120(47):e2300308120. doi: 10.1073/pnas.2300308120. Epub 2023 Nov 17. Proc Natl Acad Sci U S A. 2023. PMID: 37976261 Free PMC article.
-
A critical smn threshold in mice dictates onset of an intermediate spinal muscular atrophy phenotype associated with a distinct neuromuscular junction pathology.Neuromuscul Disord. 2012 Mar;22(3):263-76. doi: 10.1016/j.nmd.2011.09.007. Epub 2011 Nov 8. Neuromuscul Disord. 2012. PMID: 22071333
-
Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene.J Neurosci. 2010 Sep 8;30(36):12005-19. doi: 10.1523/JNEUROSCI.2208-10.2010. J Neurosci. 2010. PMID: 20826664 Free PMC article.
-
At the "junction" of spinal muscular atrophy pathogenesis: the role of neuromuscular junction dysfunction in SMA disease progression.Curr Mol Med. 2013 Aug;13(7):1160-74. doi: 10.2174/15665240113139990044. Curr Mol Med. 2013. PMID: 23514457 Review.
-
Beyond Motor Neurons in Spinal Muscular Atrophy: A Focus on Neuromuscular Junction.Int J Mol Sci. 2024 Jul 3;25(13):7311. doi: 10.3390/ijms25137311. Int J Mol Sci. 2024. PMID: 39000416 Free PMC article. Review.
Cited by
-
CDK5 promotes renal tubulointerstitial fibrosis in diabetic nephropathy via ERK1/2/PPARγ pathway.Oncotarget. 2016 Jun 14;7(24):36510-36528. doi: 10.18632/oncotarget.9058. Oncotarget. 2016. PMID: 27145370 Free PMC article.
-
Impairment of the neurotrophic signaling hub B-Raf contributes to motoneuron degeneration in spinal muscular atrophy.Proc Natl Acad Sci U S A. 2021 May 4;118(18):e2007785118. doi: 10.1073/pnas.2007785118. Proc Natl Acad Sci U S A. 2021. PMID: 33931501 Free PMC article.
-
Spinal muscular atrophy: From approved therapies to future therapeutic targets for personalized medicine.Cell Rep Med. 2021 Jul 21;2(7):100346. doi: 10.1016/j.xcrm.2021.100346. eCollection 2021 Jul 20. Cell Rep Med. 2021. PMID: 34337562 Free PMC article. Review.
-
Evaluation of putative CSF biomarkers in paediatric spinal muscular atrophy (SMA) patients before and during treatment with nusinersen.J Cell Mol Med. 2021 Sep;25(17):8419-8431. doi: 10.1111/jcmm.16802. Epub 2021 Jul 27. J Cell Mol Med. 2021. PMID: 34312963 Free PMC article.
-
Modeling Spinal Muscular Atrophy in Zebrafish: Current Advances and Future Perspectives.Int J Mol Sci. 2024 Feb 6;25(4):1962. doi: 10.3390/ijms25041962. Int J Mol Sci. 2024. PMID: 38396640 Free PMC article. Review.
References
-
- Akten B, Kye MJ, Hao le T, Wertz MH, Singh S, Nie D, Huang J, Merianda TT, Twiss JL, Beattie CE, Steen JA, Sahin M. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc Natl Acad Sci U S A. 2011;108:10337–10342. doi: 10.1073/pnas.1104928108. - DOI - PMC - PubMed
-
- Bevan AK, Hutchinson KR, Foust KD, Braun L, McGovern VL, Schmelzer L, Ward JG, Petruska JC, Lucchesi PA, Burghes AH, Kaspar BK. Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
