A new CRB1 rat mutation links Müller glial cells to retinal telangiectasia
- PMID: 25878282
- PMCID: PMC4397606
- DOI: 10.1523/JNEUROSCI.3412-14.2015
A new CRB1 rat mutation links Müller glial cells to retinal telangiectasia
Abstract
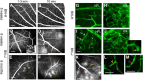
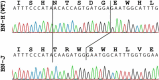
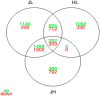
We have identified and characterized a spontaneous Brown Norway from Janvier rat strain (BN-J) presenting a progressive retinal degeneration associated with early retinal telangiectasia, neuronal alterations, and loss of retinal Müller glial cells resembling human macular telangiectasia type 2 (MacTel 2), which is a retinal disease of unknown cause. Genetic analyses showed that the BN-J phenotype results from an autosomal recessive indel novel mutation in the Crb1 gene, causing dislocalization of the protein from the retinal Müller glia (RMG)/photoreceptor cell junction. The transcriptomic analyses of primary RMG cultures allowed identification of the dysregulated pathways in BN-J rats compared with wild-type BN rats. Among those pathways, TGF-β and Kit Receptor Signaling, MAPK Cascade, Growth Factors and Inflammatory Pathways, G-Protein Signaling Pathways, Regulation of Actin Cytoskeleton, and Cardiovascular Signaling were found. Potential molecular targets linking RMG/photoreceptor interaction with the development of retinal telangiectasia are identified. This model can help us to better understand the physiopathologic mechanisms of MacTel 2 and other retinal diseases associated with telangiectasia.
Keywords: adherens junction; disease model; genetics; microcirculation; retinal blood vessels; retinal degeneration.
Copyright © 2015 the authors 0270-6474/15/356093-14$15.00/0.
Figures








References
-
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. - PubMed
-
- Charbel Issa P, Finger RP, Kruse K, Baumüller S, Scholl HP, Holz FG. Monthly ranibizumab for nonproliferative macular telangiectasia type 2: a 12-month prospective study. Am J Ophthalmol. 2011;151:876–886.e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
