Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity
- PMID: 25878293
- PMCID: PMC4397612
- DOI: 10.1523/JNEUROSCI.4137-14.2015
Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity
Abstract
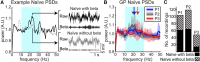
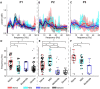
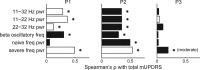
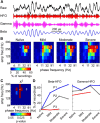
While beta oscillations often occur within the parkinsonian basal ganglia, how these oscillations emerge from a naive state and change with disease severity is not clear. To address this question, a progressive, nonhuman primate model of Parkinson's disease was developed using staged injections of MPTP. Within each parkinsonian state (naive, mild, moderate, and severe), spontaneous local field potentials were recorded throughout the sensorimotor globus pallidus. In the naive state, beta oscillations (11-32 Hz) occurred in half of the recordings, indicating spontaneous beta oscillations in globus pallidus are not pathognomonic. Mild and moderate states were characterized by a narrower distribution of beta frequencies that shifted toward the 8-15 Hz range. Additionally, coupling between the phase of beta and the amplitude of high-frequency oscillations (256-362 Hz) emerged in the mild state and increased with severity. These findings provide a novel mechanistic framework to understand how progressive loss of dopamine translates into abnormal information processing in the pallidum through alterations in oscillatory activity. The results suggest that rather than the emergence of oscillatory activity in one frequency spectrum or the other, parkinsonian motor signs may relate more to the development of altered coupling across multiple frequency spectrums.
Keywords: Parkinson's disease; globus pallidus; local field potential; oscillations; phase-amplitude coupling.
Copyright © 2015 the authors 0270-6474/15/356231-10$15.00/0.
Figures

References
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. - PubMed
-
- Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord. 1998;13(Suppl 3):29–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
