POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer
- PMID: 25878334
- PMCID: PMC4627582
- DOI: 10.1158/1078-0432.CCR-15-0057
POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer
Abstract
Purpose: Recent studies have shown that 7% to 12% of endometrial cancers are ultramutated due to somatic mutation in the proofreading exonuclease domain of the DNA replicase POLE. Interestingly, these tumors have an excellent prognosis. In view of the emerging data linking mutation burden, immune response, and clinical outcome in cancer, we investigated whether POLE-mutant endometrial cancers showed evidence of increased immunogenicity.
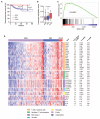
Experimental design: We examined immune infiltration and activation according to tumor POLE proofreading mutation in a molecularly defined endometrial cancer cohort including 47 POLE-mutant tumors. We sought to confirm our results by analysis of RNAseq data from the TCGA endometrial cancer series and used the same series to examine whether differences in immune infiltration could be explained by an enrichment of immunogenic neoepitopes in POLE-mutant endometrial cancers.
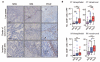
Results: Compared with other endometrial cancers, POLE mutants displayed an enhanced cytotoxic T-cell response, evidenced by increased numbers of CD8(+) tumor-infiltrating lymphocytes and CD8A expression, enrichment for a tumor-infiltrating T-cell gene signature, and strong upregulation of the T-cell cytotoxic differentiation and effector markers T-bet, Eomes, IFNG, PRF, and granzyme B. This was accompanied by upregulation of T-cell exhaustion markers, consistent with chronic antigen exposure. In silico analysis confirmed that POLE-mutant cancers are predicted to display more antigenic neoepitopes than other endometrial cancers, providing a potential explanation for our findings.
Conclusions: Ultramutated POLE proofreading-mutant endometrial cancers are characterized by a robust intratumoral T-cell response, which correlates with, and may be caused by an enrichment of antigenic neopeptides. Our study provides a plausible mechanism for the excellent prognosis of these cancers.
©2015 American Association for Cancer Research.
Figures


References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. - PubMed
-
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–e278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

