Oxygen-Loaded Nanodroplets Effectively Abrogate Hypoxia Dysregulating Effects on Secretion of MMP-9 and TIMP-1 by Human Monocytes
- PMID: 25878404
- PMCID: PMC4386605
- DOI: 10.1155/2015/964838
Oxygen-Loaded Nanodroplets Effectively Abrogate Hypoxia Dysregulating Effects on Secretion of MMP-9 and TIMP-1 by Human Monocytes
Abstract
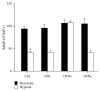
Monocytes play a key role in the inflammatory stage of the healing process. To allow monocyte migration to injured tissues, the balances between secreted matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) must be finely modulated. However, a reduction of blood supply and local oxygen tension can modify the phenotype of immune cells. Intriguingly, hypoxia might be targeted by new effective oxygenating devices such as 2H,3H-decafluoropentane- (DFP-) based oxygen-loaded nanodroplets (OLNs). Here, hypoxia effects on gelatinase/TIMP release from human peripheral monocytes were investigated, and the therapeutic potential of dextran-shelled OLNs was evaluated. Normoxic monocytes constitutively released ~500 ng/mL MMP-9, ~1.3 ng/mL TIMP-1, and ~0.6 ng/mL TIMP-2 proteins. MMP-2 was not detected. After 24 hours, hypoxia significantly altered MMP-9/TIMP-1 balance by reducing MMP-9 and increasing TIMP-1, without affecting TIMP-2 secretion. Interestingly OLNs, not displaying toxicity to human monocytes after cell internalization, effectively counteracted hypoxia, restoring a normoxia-like MMP-9/TIMP-1 ratio. The action of OLNs was specifically dependent on time-sustained oxygen diffusion up to 24 h from their DFP-based core. Therefore, OLNs appear as innovative, nonconventional, cost-effective, and nontoxic therapeutic tools, to be potentially employed to restore the physiological invasive phenotype of immune cells in hypoxia-associated inflammation.
Figures




Similar articles
-
Dextran-shelled oxygen-loaded nanodroplets reestablish a normoxia-like pro-angiogenic phenotype and behavior in hypoxic human dermal microvascular endothelium.Toxicol Appl Pharmacol. 2015 Nov 1;288(3):330-8. doi: 10.1016/j.taap.2015.08.005. Epub 2015 Aug 12. Toxicol Appl Pharmacol. 2015. PMID: 26276311
-
Chitosan-shelled oxygen-loaded nanodroplets abrogate hypoxia dysregulation of human keratinocyte gelatinases and inhibitors: New insights for chronic wound healing.Toxicol Appl Pharmacol. 2015 Aug 1;286(3):198-206. doi: 10.1016/j.taap.2015.04.015. Epub 2015 Apr 30. Toxicol Appl Pharmacol. 2015. PMID: 25937238
-
Effects of oxygen tension and dextran-shelled/2H,3H-decafluoropentane-cored oxygen-loaded nanodroplets on secretion of gelatinases and their inhibitors in term human placenta.Biosci Biotechnol Biochem. 2016;80(3):466-72. doi: 10.1080/09168451.2015.1095068. Epub 2015 Nov 2. Biosci Biotechnol Biochem. 2016. PMID: 26523859
-
Protein tyrosine kinase and p38 MAP kinase pathways are involved in stimulation of matrix metalloproteinase-9 by TNF-alpha in human monocytes.Immunol Lett. 2006 Jul 15;106(1):34-41. doi: 10.1016/j.imlet.2006.04.003. Epub 2006 May 4. Immunol Lett. 2006. PMID: 16720051
-
Electronegative LDL induces MMP-9 and TIMP-1 release in monocytes through CD14 activation: Inhibitory effect of glycosaminoglycan sulodexide.Biochim Biophys Acta Mol Basis Dis. 2018 Dec;1864(12):3559-3567. doi: 10.1016/j.bbadis.2018.09.022. Epub 2018 Sep 19. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 30254012
Cited by
-
Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies.Pharmaceutics. 2019 Aug 11;11(8):403. doi: 10.3390/pharmaceutics11080403. Pharmaceutics. 2019. PMID: 31405229 Free PMC article. Review.
-
The therapeutic effect in gliomas of nanobubbles carrying siRNA combined with ultrasound-targeted destruction.Int J Nanomedicine. 2018 Oct 24;13:6791-6807. doi: 10.2147/IJN.S164760. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30425489 Free PMC article.
-
Antimicrobial oxygen-loaded nanobubbles as promising tools to promote wound healing in hypoxic human keratinocytes.Toxicol Rep. 2022 Jan 28;9:154-162. doi: 10.1016/j.toxrep.2022.01.005. eCollection 2022. Toxicol Rep. 2022. PMID: 35145879 Free PMC article.
-
Antibacterial and Antifungal Efficacy of Medium and Low Weight Chitosan-Shelled Nanodroplets for the Treatment of Infected Chronic Wounds.Int J Nanomedicine. 2022 Apr 14;17:1725-1739. doi: 10.2147/IJN.S345553. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35444418 Free PMC article.
-
Comparative Evaluation of Different Chitosan Species and Derivatives as Candidate Biomaterials for Oxygen-Loaded Nanodroplet Formulations to Treat Chronic Wounds.Mar Drugs. 2021 Feb 15;19(2):112. doi: 10.3390/md19020112. Mar Drugs. 2021. PMID: 33672056 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

