Significant improvement of biocompatibility of polypropylene mesh for incisional hernia repair by using poly-ε-caprolactone nanofibers functionalized with thrombocyte-rich solution
- PMID: 25878497
- PMCID: PMC4388102
- DOI: 10.2147/IJN.S77816
Significant improvement of biocompatibility of polypropylene mesh for incisional hernia repair by using poly-ε-caprolactone nanofibers functionalized with thrombocyte-rich solution
Abstract
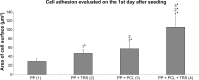
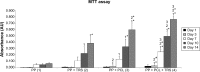
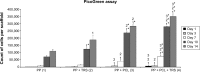
Incisional hernia is the most common postoperative complication, affecting up to 20% of patients after abdominal surgery. Insertion of a synthetic surgical mesh has become the standard of care in ventral hernia repair. However, the implementation of a mesh does not reduce the risk of recurrence and the onset of hernia recurrence is only delayed by 2-3 years. Nowadays, more than 100 surgical meshes are available on the market, with polypropylene the most widely used for ventral hernia repair. Nonetheless, the ideal mesh does not exist yet; it still needs to be developed. Polycaprolactone nanofibers appear to be a suitable material for different kinds of cells, including fibroblasts, chondrocytes, and mesenchymal stem cells. The aim of the study reported here was to develop a functionalized scaffold for ventral hernia regeneration. We prepared a novel composite scaffold based on a polypropylene surgical mesh functionalized with poly-ε-caprolactone (PCL) nanofibers and adhered thrombocytes as a natural source of growth factors. In extensive in vitro tests, we proved the biocompatibility of PCL nanofibers with adhered thrombocytes deposited on a polypropylene mesh. Compared with polypropylene mesh alone, this composite scaffold provided better adhesion, growth, metabolic activity, proliferation, and viability of mouse fibroblasts in all tests and was even better than a polypropylene mesh functionalized with PCL nanofibers. The gradual release of growth factors from biocompatible nanofiber-modified scaffolds seems to be a promising approach in tissue engineering and regenerative medicine.
Keywords: growth factors; hernia regeneration; in vitro; nanofibers; polypropylene mesh.
Figures




Similar articles
-
A polypropylene mesh modified with poly-ε-caprolactone nanofibers in hernia repair: large animal experiment.Int J Nanomedicine. 2018 May 28;13:3129-3143. doi: 10.2147/IJN.S159480. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29881270 Free PMC article.
-
Abdominal closure reinforcement by using polypropylene mesh functionalized with poly-ε-caprolactone nanofibers and growth factors for prevention of incisional hernia formation.Int J Nanomedicine. 2014 Jul 9;9:3263-77. doi: 10.2147/IJN.S63095. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25031534 Free PMC article.
-
Dynamic creep properties of a novel nanofiber hernia mesh in abdominal wall repair.Hernia. 2019 Oct;23(5):1009-1015. doi: 10.1007/s10029-019-01940-w. Epub 2019 Apr 5. Hernia. 2019. PMID: 30953212
-
Deep seroma after incisional hernia repair. Case reports and review of the literature.Ann Ital Chir. 2015 May 12;86(ePub):S2239253X15022938. Ann Ital Chir. 2015. PMID: 26007706 Review.
-
[The problem of mesh shrinkage in laparoscopic incisional hernia repair].Zentralbl Chir. 2009 Jun;134(3):209-13. doi: 10.1055/s-0028-1098779. Epub 2009 Jun 17. Zentralbl Chir. 2009. PMID: 19536713 Review. German.
Cited by
-
Personalized additive manufacturing of devices for the management of enteroatmospheric fistulas.Bioeng Transl Med. 2023 Sep 26;8(6):e10583. doi: 10.1002/btm2.10583. eCollection 2023 Nov. Bioeng Transl Med. 2023. PMID: 38023715 Free PMC article.
-
In-vitro Characterization of a Hernia Mesh Featuring a Nanostructured Coating.Front Bioeng Biotechnol. 2021 Jan 20;8:589223. doi: 10.3389/fbioe.2020.589223. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33553112 Free PMC article.
-
Effects of polymer-based, silver nanoparticle-coated silicone splints on the nasal mucosa of rats.Eur Arch Otorhinolaryngol. 2017 Mar;274(3):1535-1541. doi: 10.1007/s00405-016-4394-6. Epub 2016 Nov 18. Eur Arch Otorhinolaryngol. 2017. PMID: 27864671
-
Using a bio-scanner and 3D printing to create an innovative custom made approach for the management of complex entero-atmospheric fistulas.Sci Rep. 2020 Nov 16;10(1):19862. doi: 10.1038/s41598-020-74213-7. Sci Rep. 2020. PMID: 33199726 Free PMC article.
-
Surface treatment of artificial implants with hybrid nanolayers: results of antibacterial tests, leachates and scanning electron microscope analysis.Ann Surg Treat Res. 2024 Aug;107(2):108-119. doi: 10.4174/astr.2024.107.2.108. Epub 2024 Jul 30. Ann Surg Treat Res. 2024. PMID: 39139833 Free PMC article.
References
-
- Sugerman HJ, Kellum JM, Jr, Reines HD, DeMaria EJ, Newsome HH, Lowry JW. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171(1):80–84. - PubMed
-
- Höer J, Lawong G, Klinge U, Schumpelick V. Factors influencing the development of incisional hernia. A retrospective study of 2,983 laparotomy patients over a period of 10 years. Chirurg. 2002;73(5):474–480. German. - PubMed
-
- Vrijland WW, Bonthuis F, Steyerberg EW, Marquet RL, Jeekel J, Bonjer HJ. Peritoneal adhesions to prosthetic materials: choice of mesh for incisional hernia repair. Surg Endosc. 2000;14(10):960–963. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

