Zinc in gut-brain interaction in autism and neurological disorders
- PMID: 25878905
- PMCID: PMC4386645
- DOI: 10.1155/2015/972791
Zinc in gut-brain interaction in autism and neurological disorders
Abstract
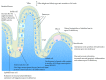
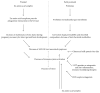
A growing amount of research indicates that abnormalities in the gastrointestinal (GI) system during development might be a common factor in multiple neurological disorders and might be responsible for some of the shared comorbidities seen among these diseases. For example, many patients with Autism Spectrum Disorder (ASD) have symptoms associated with GI disorders. Maternal zinc status may be an important factor given the multifaceted effect of zinc on gut development and morphology in the offspring. Zinc status influences and is influenced by multiple factors and an interdependence of prenatal and early life stress, immune system abnormalities, impaired GI functions, and zinc deficiency can be hypothesized. In line with this, systemic inflammatory events and prenatal stress have been reported to increase the risk for ASD. Thus, here, we will review the current literature on the role of zinc in gut formation, a possible link between gut and brain development in ASD and other neurological disorders with shared comorbidities, and tie in possible effects on the immune system. Based on these data, we present a novel model outlining how alterations in the maternal zinc status might pathologically impact the offspring leading to impairments in brain functions later in life.
Figures


Similar articles
-
Pathophysiology of autism spectrum disorders: revisiting gastrointestinal involvement and immune imbalance.World J Gastroenterol. 2014 Aug 7;20(29):9942-51. doi: 10.3748/wjg.v20.i29.9942. World J Gastroenterol. 2014. PMID: 25110424 Free PMC article. Review.
-
Gastrointestinal issues in autism spectrum disorder.Harv Rev Psychiatry. 2014 Mar-Apr;22(2):104-11. doi: 10.1097/HRP.0000000000000029. Harv Rev Psychiatry. 2014. PMID: 24614765 Review.
-
Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management.Eur J Pharmacol. 2011 Sep;668 Suppl 1:S70-80. doi: 10.1016/j.ejphar.2011.07.013. Epub 2011 Jul 27. Eur J Pharmacol. 2011. PMID: 21810417 Review.
-
Autism and nutrition: the role of the gut-brain axis.Nutr Res Rev. 2014 Dec;27(2):199-214. doi: 10.1017/S0954422414000110. Epub 2014 Jul 8. Nutr Res Rev. 2014. PMID: 25004237 Review.
-
A systematic review of gut-immune-brain mechanisms in Autism Spectrum Disorder.Dev Psychobiol. 2019 Jul;61(5):752-771. doi: 10.1002/dev.21803. Epub 2018 Dec 6. Dev Psychobiol. 2019. PMID: 30523646
Cited by
-
Impact of Zinc Transport Mechanisms on Embryonic and Brain Development.Nutrients. 2022 Jun 17;14(12):2526. doi: 10.3390/nu14122526. Nutrients. 2022. PMID: 35745255 Free PMC article. Review.
-
Immune Remodeling during Aging and the Clinical Significance of Immunonutrition in Healthy Aging.Aging Dis. 2024 Aug 1;15(4):1588-1601. doi: 10.14336/AD.2023.0923. Aging Dis. 2024. PMID: 37815906 Free PMC article. Review.
-
Nutritional status in irritable bowel syndrome: A North American population-based study.JGH Open. 2020 Feb 12;4(4):656-662. doi: 10.1002/jgh3.12311. eCollection 2020 Aug. JGH Open. 2020. PMID: 32782953 Free PMC article.
-
A 16-Year Cohort Analysis of Autism Spectrum Disorder-Associated Morbidity in a Pediatric Population.Front Psychiatry. 2018 Nov 29;9:635. doi: 10.3389/fpsyt.2018.00635. eCollection 2018. Front Psychiatry. 2018. PMID: 30555361 Free PMC article.
-
Abnormal Levels of Metal Micronutrients and Autism Spectrum Disorder: A Perspective Review.Front Mol Neurosci. 2020 Dec 10;13:586209. doi: 10.3389/fnmol.2020.586209. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33362464 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

