Rare Manifestation of a c.290 C>T, p.Gly97Glu VCP Mutation
- PMID: 25878907
- PMCID: PMC4386706
- DOI: 10.1155/2015/239167
Rare Manifestation of a c.290 C>T, p.Gly97Glu VCP Mutation
Abstract
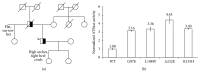
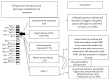
Introduction. The valosin-containing protein (VCP) regulates several distinct cellular processes. Consistent with this, VCP mutations manifest variable clinical phenotypes among and within families and are a diagnostic challenge. Methods. A 60-year-old man who played ice hockey into his 50's was evaluated by electrodiagnostics, muscle biopsy, and molecular genetics. Results. With long-standing pes cavus and toe walking, our patient developed progressive weakness, cramps, memory loss, and paresthesias at age 52. An axonal sensorimotor neuropathy was found upon repeated testing at age 58. Neuropathic histopathology was present in the quadriceps, and exome sequencing revealed the VCP mutation c.290 C>T, p.Gly97Glu. Conclusions. Our patient reflects the clinical heterogeneity of VCP mutations, as his neurological localization is a spectrum between a lower motor neuron disorder and a hereditary axonal peripheral neuropathy such as CMT2. Our case demonstrates a rare manifestation of the c.290 C>T, pGly97Glu VCP mutation.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

