Triclosan Computational Conformational Chemistry Analysis for Antimicrobial Properties in Polymers
- PMID: 25879080
- PMCID: PMC4394635
Triclosan Computational Conformational Chemistry Analysis for Antimicrobial Properties in Polymers
Abstract
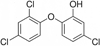
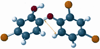
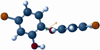
Triclosan is a diphenyl ether antimicrobial that has been analyzed by computational conformational chemistry for an understanding of Mechanomolecular Theory. Subsequent energy profile analysis combined with easily seen three-dimensional chemistry structure models for the nonpolar molecule Triclosan show how single bond rotations can alternate rapidly at a polar and nonpolar interface. Bond rotations for the center ether oxygen atom of the two aromatic rings then expose or hide nonbonding lone-pair electrons for the oxygen atom depending on the polar nature of the immediate local molecular environment. Rapid bond movements can subsequently produce fluctuations as vibration energy. Consequently, related mechanical molecular movements calculated as energy relationships by forces acting through different bond positions can help improve on current Mechanomolecular Theory. A previous controversy reported as a discrepancy in literature contends for a possible bacterial resistance from Triclosan antimicrobial. However, findings in clinical settings have not reported a single case for Triclosan bacterial resistance in over 40 years that has been documented carefully in government reports. As a result, Triclosan is recommended whenever there is a health benefit consistent with a number of approvals for use of Triclosan in healthcare devices. Since Triclosan is the most researched antimicrobial ever, literature meta analysis with computational chemistry can best describe new molecular conditions that were previously impossible by conventional chemistry methods. Triclosan vibrational energy can now explain the molecular disruption of bacterial membranes. Further, Triclosan mechanomolecular movements help illustrate use in polymer matrix composites as an antimicrobial with two new additive properties as a toughening agent to improve matrix fracture toughness from microcracking and a hydrophobic wetting agent to help incorporate strengthening fibers. Interrelated Mechanomolecular Theory by oxygen atom bond rotations or a nitrogen-type pyramidal inversion can be shown to produce energy at a polar and nonpolar boundary condition to better make clear membrane transport of other molecules, cell recognition/signaling/defense and enzyme molecular "mixing" action.
Keywords: Triclosan; antimicrobial; bonds; conformational chemistry; molecular entanglement; polar and nonpolar; rotation.
Conflict of interest statement
Conflict of interest: No conflicts declared.
Figures


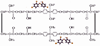
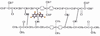

References
-
- McMurry J. Organic Chemistry. 6th Edition. Cole, Belmont, CA: Thompson Brooks; 2004.
-
- Brown WH, Foote CS, Everson BL, Anslyn EV. Organic Chemistry. 5th Edition. Belmont, CA: Brooks/Cole; 2009.
-
- Zukerman DM. Statistical Physics of Biomolecules an Introduction. Boca Raton: CRC Press Taylor & Francis Group; 2010.
-
- Tinoco I, Sauer K, Wang JC, Puglisi JD, Harbison G, Rovnyak D. Physical Chemistry Principles and Applications in Biological Sciences. 5th Edition. New York: Pearson; 2013.
-
- Petersen RC. Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering. Int. J. Comp. Mat. Sci. Eng. 2014;3(1):1450003. (48 pages) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295723/ - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
