Comparative Effectiveness of Post-Discharge Strategies for Hospitalized Smokers: study protocol for the Helping HAND 2 randomized controlled trial
- PMID: 25879193
- PMCID: PMC4328622
- DOI: 10.1186/s12889-015-1484-0
Comparative Effectiveness of Post-Discharge Strategies for Hospitalized Smokers: study protocol for the Helping HAND 2 randomized controlled trial
Abstract
Background: Smoking cessation interventions for hospitalized smokers are effective in promoting smoking cessation, but only if the tobacco dependence treatment continues after the patient leaves the hospital. Sustaining tobacco dependence treatment after hospital discharge is a challenge for health care systems. Our previous single-site randomized controlled trial demonstrated the effectiveness of an intervention that facilitated the delivery of comprehensive tobacco cessation treatment, including both medication and counseling, after hospital discharge. We subsequently streamlined the intervention model to increase its potential for dissemination. This new model is being tested in a larger multi-site trial with broader eligibility criteria in order to enroll a more representative sample of hospitalized smokers. This paper describes the trial design and contrasts it with the earlier study.
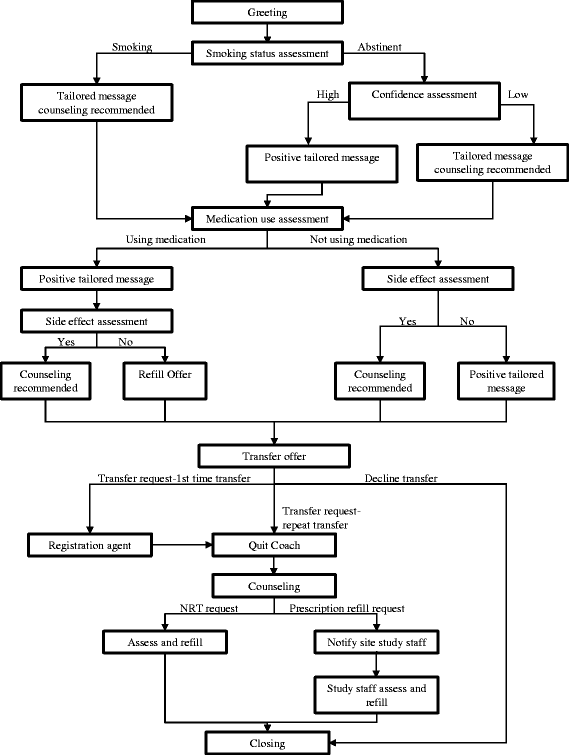
Methods/design: A 2-arm, 3-site randomized controlled trial is testing the hypothesis that a multi-component Sustained Care intervention is more effective than Standard Care in helping hospitalized cigarette smokers stop smoking after hospital discharge. The trial enrolls adult daily cigarette smokers who are admitted to 1 of 3 participating hospitals in Massachusetts or Pennsylvania. Participants receive the same smoking cessation intervention in the hospital. They are randomly assigned to receive either Standard Care or Sustained Care after hospital discharge. Participants in the Sustained Care arm receive a free 3-month supply of FDA-approved smoking cessation medication and 5 interactive voice response calls that provide tailored motivational messages, medication refills, and access to a live tobacco treatment counselor. Participants in the Standard Care arm receive a smoking cessation medication recommendation and information about community resources. Outcomes are assessed at 1, 3, and 6 months after discharge. The primary outcome is biochemically-validated tobacco abstinence for the past 7 days at 6-month follow-up. Other outcome measures include self-reported tobacco abstinence measures, use of medication and counseling after discharge, hospital readmissions, and program cost-effectiveness.
Discussion: We adapted a proven intervention for hospitalized smokers to enhance its potential for dissemination and are testing it in a multi-site trial. Study enrollment data suggests that the trial achieved the goal of recruiting a broader sample of hospitalized smokers.
Trial registration: Comparative Effectiveness of Post-Discharge Strategies for Hospitalized Smokers (Helping HAND2) NCT01714323. Registered October 22, 2012.
Figures
References
-
- U.S. Department of Health and Human Services . The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. - PubMed
-
- Centers for Disease Control and Prevention Quitting smoking among adults-United States, 2001-2010. MMWR. 2011;60(44):1513–1519. - PubMed
-
- National Health Interview Survey. 2008. http://www.cdc.gov/nchs/nhis.htm. Accessed September 2014.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous