Regulation of inflammasome activation
- PMID: 25879280
- PMCID: PMC4400844
- DOI: 10.1111/imr.12296
Regulation of inflammasome activation
Abstract
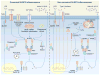
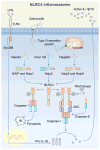
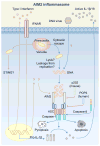
Inflammasome biology is one of the most exciting and rapidly growing areas in immunology. Over the past 10 years, inflammasomes have been recognized for their roles in the host defense against invading pathogens and in the development of cancer, auto-inflammatory, metabolic, and neurodegenerative diseases. Assembly of an inflammasome complex requires cytosolic sensing of pathogen-associated molecular patterns or danger-associated molecular patterns by a nucleotide-binding domain and leucine-rich repeat receptor (NLR) or absent in melanoma 2 (AIM2)-like receptors (ALR). NLRs and ALRs engage caspase-1, in most cases requiring the adapter protein apoptosis-associated speck-like protein containing a CARD (ASC), to catalyze proteolytic cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18 and drive pyroptosis. Recent studies indicate that caspase-8, caspase-11, IL-1R-associated kinases (IRAK), and receptor-interacting protein (RIP) kinases contribute to inflammasome functions. In addition, post-translational modifications, including ubiquitination, deubiquitination, phosphorylation, and degradation control almost every aspect of inflammasome activities. Genetic studies indicate that mutations in NLRP1, NLRP3, NLRC4, and AIM2 are linked with the development of auto-inflammatory diseases, enterocolitis, and cancer. Overall, these findings transform our understanding of the basic biology and clinical relevance of inflammasomes. In this review, we provide an overview of the latest development of inflammasome research and discuss how inflammasome activities govern health and disease.
Keywords: IL-1; NLR; caspase-1; caspase-11; caspase-8; inflammasome.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. - PubMed
-
- Bertin J, et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. The Journal of biological chemistry. 1999;274:12955–12958. - PubMed
-
- Inohara N, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. The Journal of biological chemistry. 1999;274:14560–14567. - PubMed
-
- Harton JA, Linhoff MW, Zhang J, Ting JP. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. Journal of immunology. 2002;169:4088–4093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

