CXCL13 antibody for the treatment of autoimmune disorders
- PMID: 25879435
- PMCID: PMC4329654
- DOI: 10.1186/s12865-015-0068-1
CXCL13 antibody for the treatment of autoimmune disorders
Abstract
Background: Homeostatic B Cell-Attracting chemokine 1 (BCA-1) otherwise known as CXCL13 is constitutively expressed in secondary lymphoid organs by follicular dendritic cells (FDC) and macrophages. It is the only known ligand for the CXCR5 receptor, which is expressed on mature B cells, follicular helper T cells (Tfh), Th17 cells and regulatory T (Treg) cells. Aberrant expression of CXCL13 within ectopic germinal centers has been linked to the development of autoimmune disorders (e.g. Rheumatoid Arthritis, Multiple Sclerosis, Systemic Lupus Erythematosis). We, therefore, hypothesized that antibody-mediated disruption of the CXCL13 signaling pathway would interfere with the formation of ectopic lymphoid follicles in the target organs and inhibit autoimmune disease progression. This work describes pre-clinical development of human anti-CXCL13 antibody MAb 5261 and includes therapeutic efficacy data of its mouse counterpart in murine models of autoimmunity.
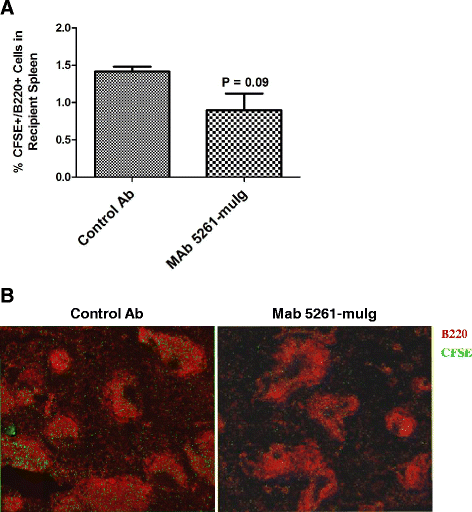
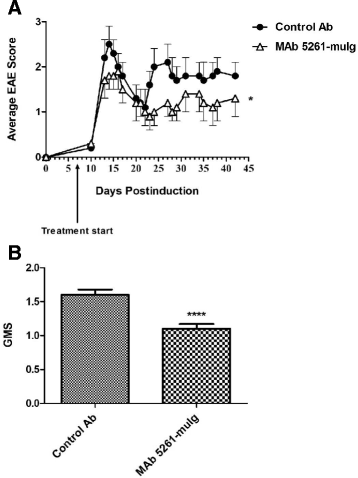
Results: We developed a human IgG1 monoclonal antibody, MAb 5261 that specifically binds to human, rodent and primate CXCL13 with an affinity of approximately 5 nM and is capable of neutralizing the activity of CXCL13 from these various species in in vitro functional assays. For in vivo studies we have engineered a chimeric antibody to contain the same human heavy and light chain variable genes along with mouse constant regions. Treatment with this antibody led to a reduction in the number of germinal centers in mice immunized with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Keyhole Limpet Hemocyanin (NP-KLH) and, in adoptive transfer studies, interfered with the trafficking of B cells to the B cell areas of mouse spleen. Furthermore, this mouse anti-CXCL13 antibody demonstrated efficacy in a mouse model of Rheumatoid arthritis (Collagen-Induced Arthritis (CIA)) and Th17-mediated murine model of Multiple Sclerosis (passively-induced Experimental Autoimmune Encephalomyelitis (EAE)).
Conclusions: We developed a novel therapeutic antibody targeting CXCL13-mediated signaling pathway for the treatment of autoimmune disorders.
Figures






Similar articles
-
Role of TFH Cells in Promoting T Helper 17-Induced Neuroinflammation.Front Immunol. 2018 Feb 27;9:382. doi: 10.3389/fimmu.2018.00382. eCollection 2018. Front Immunol. 2018. PMID: 29535739 Free PMC article.
-
Rebamipide attenuates autoimmune arthritis severity in SKG mice via regulation of B cell and antibody production.Clin Exp Immunol. 2014 Oct;178(1):9-19. doi: 10.1111/cei.12355. Clin Exp Immunol. 2014. PMID: 24749771 Free PMC article.
-
Role of the CXCL13/CXCR5 Axis in Autoimmune Diseases.Front Immunol. 2022 Mar 4;13:850998. doi: 10.3389/fimmu.2022.850998. eCollection 2022. Front Immunol. 2022. PMID: 35309354 Free PMC article. Review.
-
Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients.J Immunol. 2001 Jan 1;166(1):650-5. doi: 10.4049/jimmunol.166.1.650. J Immunol. 2001. PMID: 11123349
-
The CXCL13/CXCR5 Immune Axis in Health and Disease-Implications for Intrathecal B Cell Activities in Neuroinflammation.Cells. 2022 Aug 25;11(17):2649. doi: 10.3390/cells11172649. Cells. 2022. PMID: 36078057 Free PMC article. Review.
Cited by
-
The curious origins of angioimmunoblastic T-cell lymphoma.Curr Opin Hematol. 2016 Jul;23(4):434-43. doi: 10.1097/MOH.0000000000000261. Curr Opin Hematol. 2016. PMID: 27177312 Free PMC article. Review.
-
The Ameliorative Effect of Dexamethasone on the Development of Autoimmune Lung Injury and Mediastinal Fat-Associated Lymphoid Clusters in an Autoimmune Disease Mouse Model.Int J Mol Sci. 2022 Apr 18;23(8):4449. doi: 10.3390/ijms23084449. Int J Mol Sci. 2022. PMID: 35457267 Free PMC article.
-
Advances in Biomarker-Guided Therapy for Pediatric- and Adult-Onset Neuroinflammatory Disorders: Targeting Chemokines/Cytokines.Front Immunol. 2018 Apr 4;9:557. doi: 10.3389/fimmu.2018.00557. eCollection 2018. Front Immunol. 2018. PMID: 29670611 Free PMC article. Review.
-
Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis.Nat Rev Neurol. 2023 Aug;19(8):461-476. doi: 10.1038/s41582-023-00838-7. Epub 2023 Jul 3. Nat Rev Neurol. 2023. PMID: 37400550 Review.
-
Association of Serum CXCL13 with Intrarenal Ectopic Lymphoid Tissue Formation in Lupus Nephritis.J Immunol Res. 2016;2016:4832543. doi: 10.1155/2016/4832543. Epub 2016 Nov 20. J Immunol Res. 2016. PMID: 27990444 Free PMC article.
References
-
- Förster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

