Transiently lowering tumor necrosis factor-α synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice
- PMID: 25879458
- PMCID: PMC4352276
- DOI: 10.1186/s12974-015-0237-4
Transiently lowering tumor necrosis factor-α synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice
Abstract
Background: The treatment of traumatic brain injury (TBI) represents an unmet medical need, as no effective pharmacological treatment currently exists. The development of such a treatment requires a fundamental understanding of the pathophysiological mechanisms that underpin the sequelae resulting from TBI, particularly the ensuing neuronal cell death and cognitive impairments. Tumor necrosis factor-alpha (TNF-α) is a cytokine that is a master regulator of systemic and neuroinflammatory processes. TNF-α levels are reported to become rapidly elevated post TBI and, potentially, can lead to secondary neuronal damage.
Methods: To elucidate the role of TNF-α in TBI, particularly as a drug target, the present study evaluated (i) time-dependent TNF-α levels and (ii) markers of apoptosis and gliosis within the brain and related these to behavioral measures of 'well being' and cognition in a mouse closed head 50 g weight drop mild TBI (mTBI) model in the presence and absence of post-treatment with an experimental TNF-α synthesis inhibitor, 3,6'-dithiothalidomide.
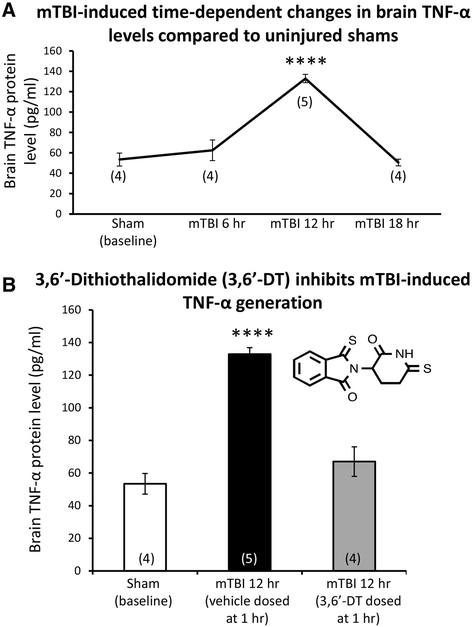
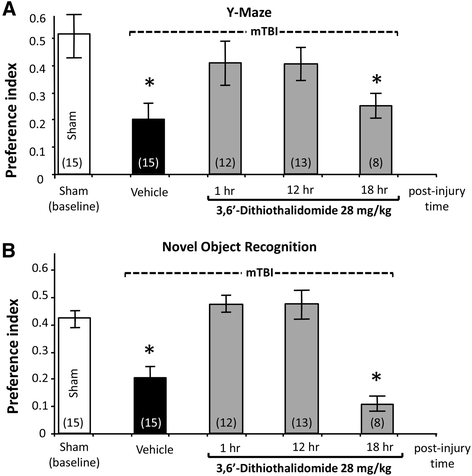
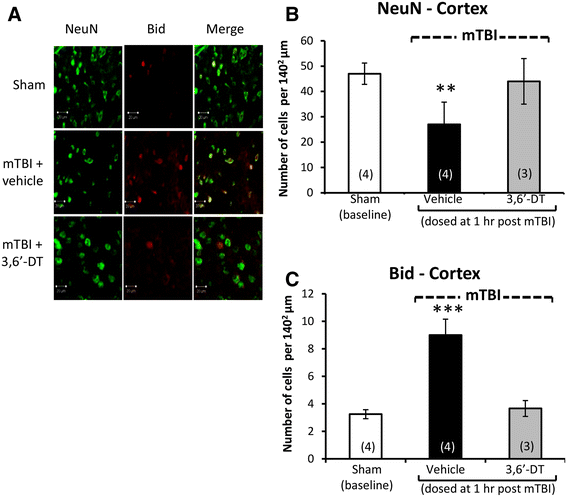
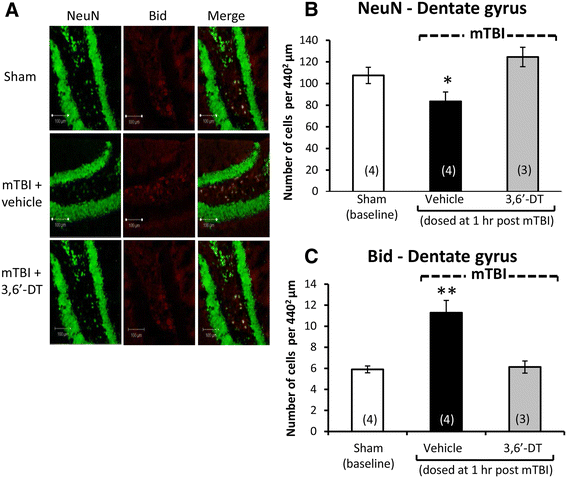
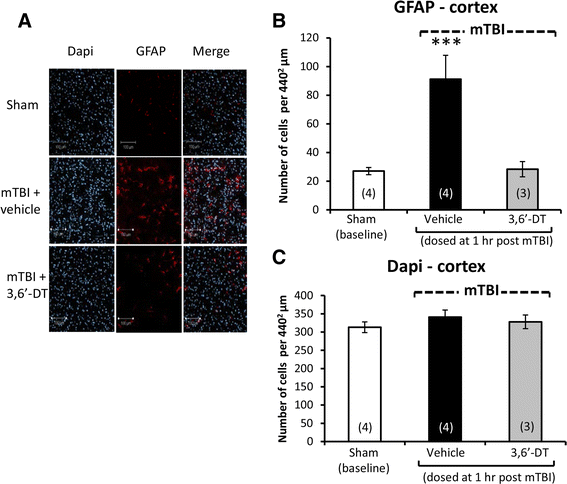
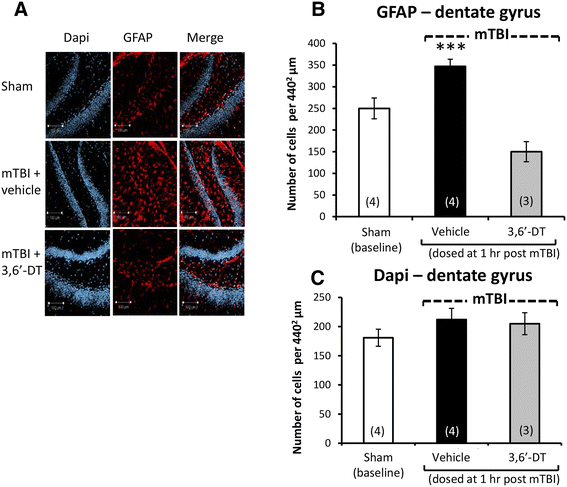
Results: mTBI elevated brain TNF-α levels, which peaked at 12 h post injury and returned to baseline by 18 h. This was accompanied by a neuronal loss and an increase in astrocyte number (evaluated by neuronal nuclei (NeuN) and glial fibrillary acidic protein (GFAP) immunostaining), as well as an elevation in the apoptotic death marker BH3-interacting domain death agonist (BID) at 72 h. Selective impairments in measures of cognition, evaluated by novel object recognition and passive avoidance paradigms - without changes in well being, were evident at 7 days after injury. A single systemic treatment with the TNF-α synthesis inhibitor 3,6'-dithiothalidomide 1 h post injury prevented the mTBI-induced TNF-α elevation and fully ameliorated the neuronal loss (NeuN), elevations in astrocyte number (GFAP) and BID, and cognitive impairments. Cognitive impairments evident at 7 days after injury were prevented by treatment as late as 12 h post mTBI but were not reversed when treatment was delayed until 18 h.
Conclusions: These results implicate that TNF-α in mTBI induced secondary brain damage and indicate that pharmacologically limiting the generation of TNF-α post mTBI may mitigate such damage, defining a time-dependent window of up to 12 h to achieve this reversal.
Figures
References
-
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–53. - PubMed
-
- Faul M, Xu L, Wald M, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

