The implementation of an organised cervical screening programme in Poland: an analysis of the adherence to European guidelines
- PMID: 25879466
- PMCID: PMC4417537
- DOI: 10.1186/s12885-015-1242-9
The implementation of an organised cervical screening programme in Poland: an analysis of the adherence to European guidelines
Abstract
Background: Well-organised quality-controlled screening can substantially reduce the burden of cervical cancer (CC). European guidelines (EuG) for quality assurance in CC screening provide guidance on all aspects of an organised screening programme. Organised CC screening in Poland was introduced in 2007. The purpose of our study was to analyse: (i) adherence of the programme to EuG; (ii) programme process and performance indicators; (iii) impact of the programme on the incidence of and mortality from CC.
Methods: Available data on the policy, structure and functioning of the Polish programme were compared with the major points of the EuG. Data on the process, and available performance indicators were drawn from the screening database and other National Health Fund (NHF) systems. Joinpoint regression was used to assess changes in CC incidence and mortality trends.
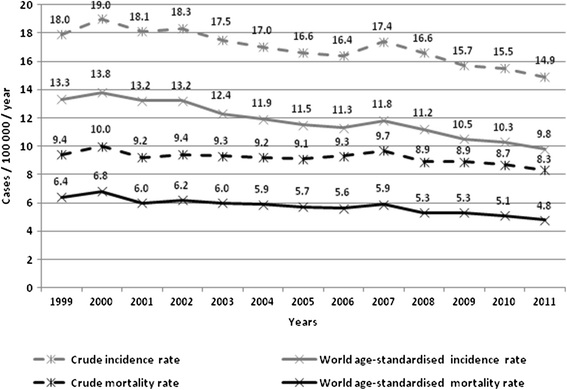
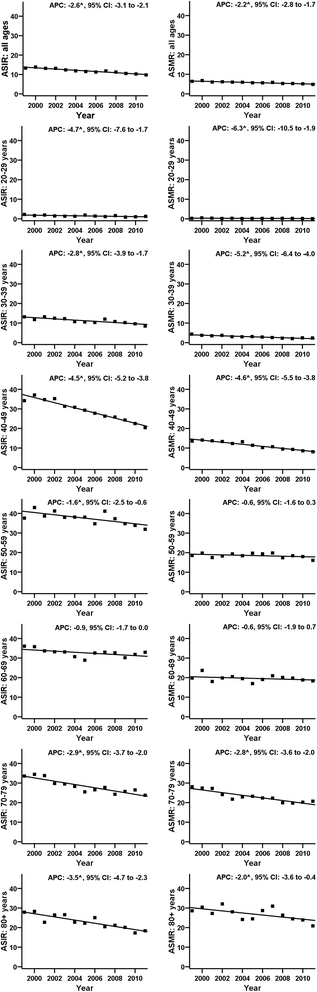
Results: The Polish programme adheres partially to EuG in terms of policy and organisation. Only a limited set of performance indicators can be calculated due to screening database incompleteness or lack of linkage between existing databases. The screening database does not include opportunistic smears collected within NHF-reimbursed or private care. The organised programme coverage rate fluctuated from 21% to 27% between 2007-2013. In 2012 the coverage reached 35% after combining both organised and opportunistic smears reimbursed by the NHF. In 2012 the number of smears reimbursed by NHF was 60% higher in opportunistic than in organised screening with significant overlap. Data from the private sector are not recorded. Depending on years, 30-50% of women referred for colposcopy/biopsy because of abnormal Pap smears were managed within the programme. The age-standardised CC incidence and mortality dropped linearly between 1999 and 2011 without evidence of a period effect.
Conclusions: The Polish organised cervical screening programme is only partially adherent to evidence-based EuG. Its implementation has not influenced the burden of CC in the country so far. Changes with special focus on increasing coverage, development of information systems and assessment of quality are required to increase programme adherence to EuG and to measure its effectiveness. Our findings may be useful to improve the Polish programme and those implemented or planned in other countries.
Figures
References
-
- Hakama M, Miller AB, Day NE. Screening for Cancer of the Uterine Cervix. From the IARC Working Group on Cervical Cancer Screening and the UICC Project Group on the Evaluation of Screening Programmes for Cancer. WHO (Geneva), IARC (Lyon), and UICC (Geneva): IARC Sci Publ; 1986. - PubMed
-
- Siebert U, Sroczynski G, Hillemanns P, Engel J, Stabenow R, Stegmaier C, et al. The German cervical cancer screening model: development and validation of a decision-analytic model for cervical cancer screening in Germany. Eur J Public Health. 2006;16:185–92. doi: 10.1093/eurpub/cki163. - DOI - PubMed
-
- Wojciechowska U, Didkowska J. Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy Rejestr Nowotworów, Centrum Onkologii - Instytut im. Marii Skłodowskiej - Curie. Available at: http://onkologia.org.pl.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

