Titration of biologically active amyloid-β seeds in a transgenic mouse model of Alzheimer's disease
- PMID: 25879692
- PMCID: PMC4399520
- DOI: 10.1038/srep09349
Titration of biologically active amyloid-β seeds in a transgenic mouse model of Alzheimer's disease
Abstract
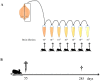
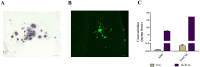
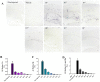
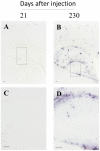
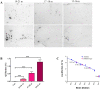
Experimental evidence in animal models suggests that misfolded Amyloid-β (Aβ) spreads in disease following a prion-like mechanism. Several properties characteristics of infectious prions have been shown for the induction of Aβ aggregates. However, a detailed titration of Aβ misfolding transmissibility and estimation of the minimum concentration of biologically active Aβ seeds able to accelerate pathological changes has not yet been performed. In this study, brain extracts from old tg2576 animals were serially diluted and intra-cerebrally injected into young subjects from the same transgenic line. Animals were sacrificed several months after treatment and brain slices were analyzed for amyloid pathology. We observed that administration of misfolded Aβ was able to significantly accelerate amyloid deposition in young mice, even when the original sample was diluted a million times. The titration curve obtained in this experiment was compared to the natural Aβ load spontaneously accumulated by these mice overtime. Our findings suggest that administration of the largest dose of Aβ seeds led to an acceleration of pathology equivalent to over a year. These results show that active Aβ seeds present in the brain can seed amyloidosis in a titratable manner, similarly as observed for infectious prions.
Figures

References
-
- Cleary J. P. et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. (2004). - PubMed
-
- Klein W. L. Synaptotoxic amyloid-beta oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer's disease? J. Alzheimers. Dis. 33 Suppl 1, S49–S65 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

