Mutagenesis of Trichoderma reesei endoglucanase I: impact of expression host on activity and stability at elevated temperatures
- PMID: 25879765
- PMCID: PMC4347658
- DOI: 10.1186/s12896-015-0118-z
Mutagenesis of Trichoderma reesei endoglucanase I: impact of expression host on activity and stability at elevated temperatures
Abstract
Background: Trichoderma reesei is a key cellulase source for economically saccharifying cellulosic biomass for the production of biofuels. Lignocellulose hydrolysis at temperatures above the optimum temperature of T. reesei cellulases (~50°C) could provide many significant advantages, including reduced viscosity at high-solids loadings, lower risk of microbial contamination during saccharification, greater compatibility with high-temperature biomass pretreatment, and faster rates of hydrolysis. These potential advantages motivate efforts to engineer T. reesei cellulases that can hydrolyze lignocellulose at temperatures ranging from 60-70°C.
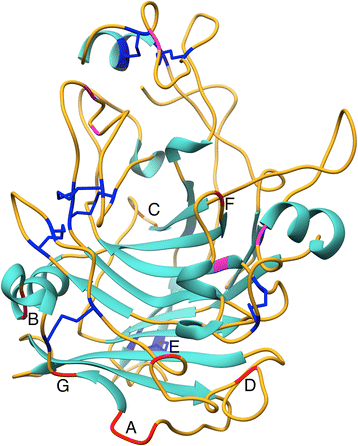
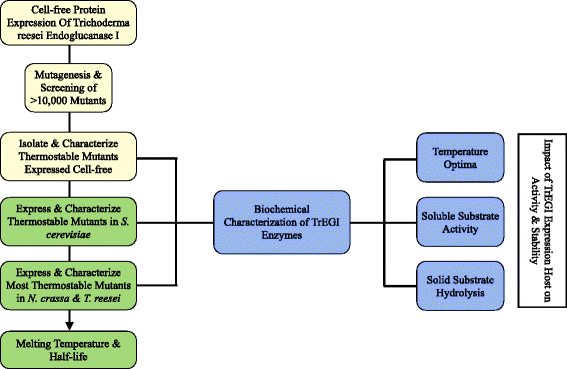
Results: A B-factor guided approach for improving thermostability was used to engineer variants of endoglucanase I (Cel7B) from T. reesei (TrEGI) that are able to hydrolyze cellulosic substrates more rapidly than the recombinant wild-type TrEGI at temperatures ranging from 50-70°C. When expressed in T. reesei, TrEGI variant G230A/D113S/D115T (G230A/D113S/D115T Tr_TrEGI) had a higher apparent melting temperature (3°C increase in Tm) and improved half-life at 60°C (t1/2 = 161 hr) than the recombinant (T. reesei host) wild-type TrEGI (t1/2 = 74 hr at 60°C, Tr_TrEGI). Furthermore, G230A/D113S/D115T Tr_TrEGI showed 2-fold improved activity compared to Tr_TrEGI at 65°C on solid cellulosic substrates, and was as efficient in hydrolyzing cellulose at 60°C as Tr_TrEGI was at 50°C. The activities and stabilities of the recombinant TrEGI enzymes followed similar trends but differed significantly in magnitude depending on the expression host (Escherichia coli cell-free, Saccharomyces cerevisiae, Neurospora crassa, or T. reesei). Compared to N.crassa-expressed TrEGI, S. cerevisiae-expressed TrEGI showed inferior activity and stability, which was attributed to the lack of cyclization of the N-terminal glutamine in Sc_TrEGI and not to differences in glycosylation. N-terminal pyroglutamate formation in TrEGI expressed in S. cerevisiae was found to be essential in elevating its activity and stability to levels similar to the T. reesei or N. crassa-expressed enzyme, highlighting the importance of this ubiquitous modification in GH7 enzymes.
Conclusion: Structure-guided evolution of T. reesei EGI was used to engineer enzymes with increased thermal stability and activity on solid cellulosic substrates. Production of TrEGI enzymes in four hosts highlighted the impact of the expression host and the role of N-terminal pyroglutamate formation on the activity and stability of TrEGI enzymes.
Figures


References
-
- Viikari L, Alapuranen M, Puranen T, Vehmaanpera J, Siika-Aho M. Thermostable enzymes in lignocellulose hydrolysis. In: Olsson L, editor. Biofuels.vol. 108. Berlin: Springer-Verlag Berlin; 2007. pp. 121–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

