miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met
- PMID: 25880599
- PMCID: PMC6496886
- DOI: 10.1111/cpr.12187
miR-454 is down-regulated in osteosarcomas and suppresses cell proliferation and invasion by directly targeting c-Met
Abstract
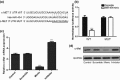
Objectives: Osteosarcoma is the most common primary bone malignancy of children and young adults. Increasing evidence has shown that microRNAs (miRNAs) are associated with cancer development, but, little is known concerning the role of miR-454 in osteosarcoma.
Materials and methods: qRT-PCR was performed to detect expression of miR-454 in osteosarcoma cell lines and tissues. To understand its role in osteosarcoma, we reintroduced expression of miR-454 in the MG-63 cell line by transfection with miR-454 mimics or inhibitors. CCK-8 assay and an invasion assay were used to detect the functional role of miR-454. Luciferase assay and western blot analysis were performed to detect the target gene of miR-454.
Results: miR-454 was found to be down-regulated in osteosarcoma tissues and cell lines. Its over-expression inhibited tumour growth and invasion and its down-regulation promoted cell proliferation and invasion. Subsequent investigation revealed that c-Met was a direct and functional target of miR-454 in osteosarcoma. Overexpression of miR-454 impaired c-Met-induced cell proliferation and invasion. Finally, miR-454 was found to be inversely correlated to c-Met expression in human osteosarcoma tissues.
Conclusions: Reduced-expression of miR-454 in osteosarcoma cells promoted tumour growth by targeting c-Met, thus miR-454 may be a potential therapy target for this tumour.
© 2015 John Wiley & Sons Ltd.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

