High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination
- PMID: 25880733
- PMCID: PMC4407781
- DOI: 10.1186/s12887-015-0359-y
High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination
Abstract
Background: Antibiotic dosing in neonates varies between countries and centres, suggesting suboptimal exposures for some neonates. We aimed to describe variations and factors influencing the variability in the dosing of frequently used antibiotics in European NICUs to help define strategies for improvement.
Methods: A sub-analysis of the European Study of Neonatal Exposure to Excipients point prevalence study was undertaken. Demographic data of neonates receiving any antibiotic on the study day within one of three two-week periods from January to June 2012, the dose, dosing interval and route of administration of each prescription were recorded. The British National Formulary for Children (BNFC) and Neofax were used as reference sources. Risk factors for deviations exceeding ±25% of the relevant BNFC dosage recommendation were identified by multivariate logistic regression analysis.
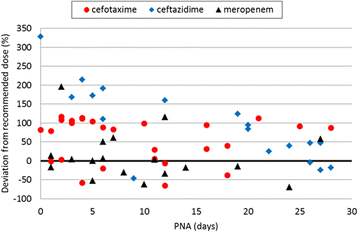
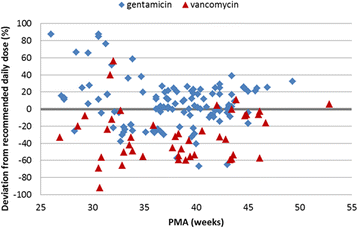
Results: In 89 NICUs from 21 countries, 586 antibiotic prescriptions for 342 infants were reported. The twelve most frequently used antibiotics - gentamicin, penicillin G, ampicillin, vancomycin, amikacin, cefotaxime, ceftazidime, meropenem, amoxicillin, metronidazole, teicoplanin and flucloxacillin - covered 92% of systemic prescriptions. Glycopeptide class, GA <32 weeks, 5(th) minute Apgar score <5 and geographical region were associated with deviation from the BNFC dosage recommendation. While the doses of penicillins exceeded recommendations, antibiotics with safety concerns followed (gentamicin) or were dosed below (vancomycin) recommendations.
Conclusions: The current lack of compliance with existing dosing recommendations for neonates needs to be overcome through the conduct of well-designed clinical trials with a limited number of antibiotics to define pharmacokinetics/pharmacodynamics, efficacy and safety in this population and by efficient dissemination of the results.
Figures


References
-
- Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H. The antibiotic resistance and prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32(6):e242–e253. doi: 10.1097/INF.0b013e318286c612. - DOI - PubMed
-
- Lass J, Kaar R, Jogi K, Varendi H, Metsvaht T, Lutsar I. Drug utilisation pattern and off-label use of medicines in Estonian neonatal units. Eur J Clin Pharmacol. 2011;67(12):1263-71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

