Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study
- PMID: 25880756
- PMCID: PMC4397697
- DOI: 10.1186/s12883-015-0305-5
Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study
Abstract
Background: Eslicarbazepine acetate (ESL, Aptiom®) is a once-daily (QD) anticonvulsant, approved as adjunctive treatment of partial-onset seizures (POS). It is extensively converted after oral administration to eslicarbazepine, and is believed to exert its effect through inhibition of voltage-gated sodium channels. The possible role of ESL as monotherapy to treat POS has not yet been established.
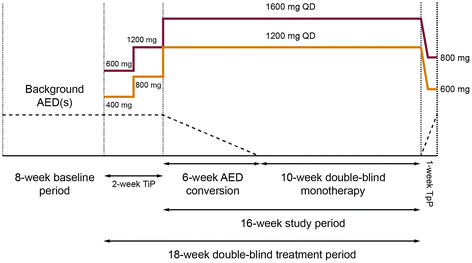
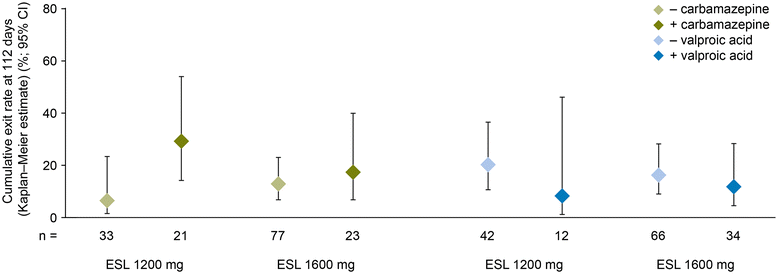
Methods: This study was an 18-week, multicenter, randomized double-blind trial of gradual conversion to ESL monotherapy in adults with POS not well controlled by 1-2 antiepileptic drugs (AEDs), using historical data as the control. The study comprised an 8-week baseline period, a 2-week titration period, a 6-week AED conversion period, a 10-week monotherapy period, and either a 1-week taper period or optional entry to an open-label extension study. The primary endpoint compared the Kaplan-Meier (KM)-estimated 112-day exit rate with a threshold value calculated from the historical controls.
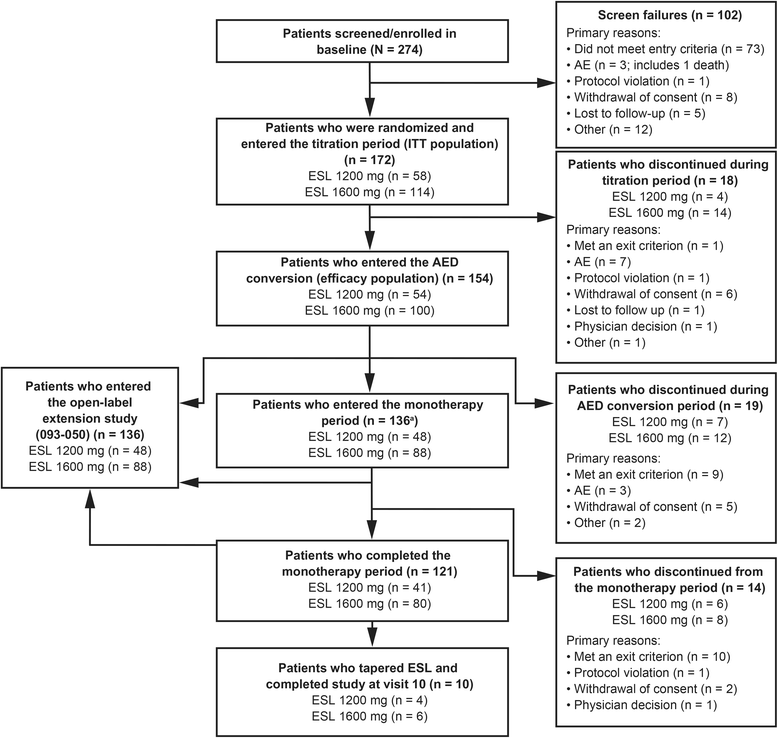
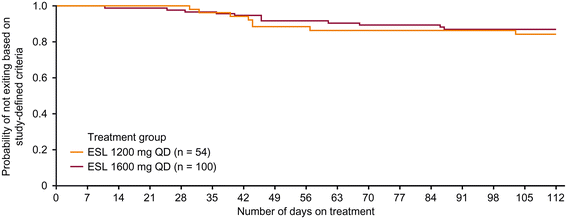
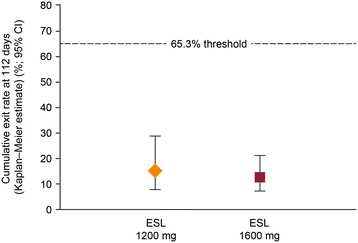
Results: There were 172 randomized patients; 154 (90%) entered the AED conversion period and 121 (70%) completed the study. The KM-estimated exit rates [confidence interval (CI)] were 15.6% [8.1-28.7%] for ESL 1200 mg, and 12.8% [7.5-21.5%] for ESL 1600 mg. The upper limits of the 95% CI KM-estimates were below the pre-specified threshold for historical control of 65.3%, indicating that ESL was efficacious in reducing seizure-related exits, compared with historical control. During the 18-week double-blind treatment period, median reductions in standardized seizure frequency occurred with ESL 1200 mg (36.1%) and ESL 1600 mg (47.5%). The responder rates (a 50% or greater reduction in seizure frequency from baseline) during the 18-week double-blind period and the monotherapy period, respectively, were 35.2% and 38.9% for ESL 1200 mg, and 46.0% and 46.0% for ESL 1600 mg. The overall adverse event profile was consistent with the known safety profile of ESL.
Conclusions: These findings indicate that ESL monotherapy (1200 and 1600 mg QD) was efficacious and well tolerated in this study.
Trial registration: NCT01091662 ; EudraCT No. 2010-018684-42.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

