Fungal cytochrome P450 monooxygenases of Fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae
- PMID: 25880760
- PMCID: PMC4387584
- DOI: 10.1186/s12934-015-0228-2
Fungal cytochrome P450 monooxygenases of Fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae
Abstract
Background: Omega hydroxy fatty acids (ω-OHFAs) are multifunctional compounds that act as the basis for the production of various industrial products with broad commercial and pharmaceutical implications. However, the terminal oxygenation of saturated or unsaturated fatty acids for the synthesis of ω-OHFAs is intricate to accomplish through chemocatalysis, due to the selectivity and controlled reactivity in C-H oxygenation reactions. Cytochrome P450, the ubiquitous enzyme is capable of catalyzing the selective terminal omega hydroxylation naturally in biological kingdom.
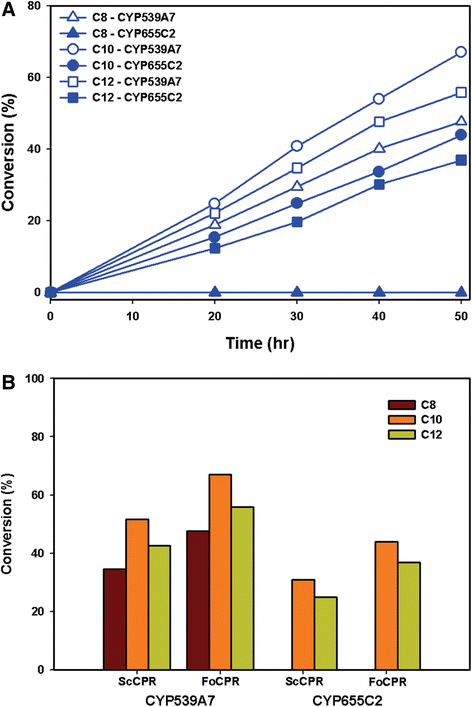
Results: To gain a deep insight on the biochemical role of fungal P450s towards the production of omega hydroxy fatty acids, two cytochrome P450 monooxygenases from Fusarium oxysporum (FoCYP), FoCYP539A7 and FoCYP655C2; were identified, cloned, and heterologously expressed in Saccharomyces cerevisiae. For the efficient production of ω-OHFAs, the S. cerevisiae was engineered to disrupt the acyl-CoA oxidase enzyme and the β-oxidation pathway inactivated (ΔPox1) S. cerevisiae mutant was generated. To elucidate the significance of the interaction of redox mechanism, FoCYPs were reconstituted with the heterologous and homologous reductase systems--S. cerevisiae CPR (ScCPR) and F. oxysporum CPR (FoCPR). To further improve the yield, the effect of pH was analyzed and the homologous FoCYP-FoCPR system efficiently hydroxylated caprylic acid, capric acid and lauric acid into their respective ω-hydroxy fatty acids with 56%, 79% and 67% conversion. Furthermore, based on computational simulations, we identified the key residues (Asn106 of FoCYP539A7 and Arg235 of FoCYP655C2) responsible for the recognition of fatty acids and demonstrated the structural insights of the active site of FoCYPs.
Conclusion: Fungal CYP monooxygenases, FoCYP539A7 and FoCYP655C2 with its homologous redox partner, FoCPR constitutes a promising catalyst due to its high regio- and stereo-selectivity in the hydroxylation of fatty acids and in the substantial production of industrially valuable ω-hydroxy fatty acids.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

