Functional interplay between MYCN, NCYM, and OCT4 promotes aggressiveness of human neuroblastomas
- PMID: 25880909
- PMCID: PMC4520635
- DOI: 10.1111/cas.12677
Functional interplay between MYCN, NCYM, and OCT4 promotes aggressiveness of human neuroblastomas
Abstract
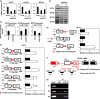
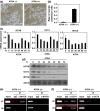
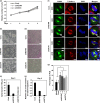
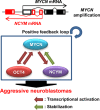
Neuroblastoma is a pediatric solid tumor that originates from embryonic neural crest cells. The MYCN gene locus is frequently amplified in unfavorable neuroblastomas, and the gene product promotes the progression of neuroblastomas. However, the molecular mechanisms by which MYCN amplification contributes to stem cell-like states of neuroblastoma remain elusive. In this study, we show that MYCN and its cis-antisense gene, NCYM, form a positive feedback loop with OCT4, a core regulatory gene maintaining a multipotent state of neural stem cells. We previously reported that NCYM is co-amplified with the MYCN gene in primary human neuroblastomas and that the gene product promotes aggressiveness of neuroblastoma by stabilization of MYCN. In 36 MYCN-amplified primary human neuroblastomas, OCT4 mRNA expression was associated with unfavorable prognosis and was correlated with that of NCYM. The OCT4 protein induced both NCYM and MYCN in human neuroblastoma cells, whereas NCYM stabilized MYCN to induce OCT4 and stem cell-related genes, including NANOG, SOX2, and LIN28. In sharp contrast to MYCN, enforced expression of c-MYC did not enhance OCT4 expression in human neuroblastoma cells. All-trans retinoic acid treatment reduced MYCN, NCYM, and OCT4 expression, accompanied by the decreased amount of OCT4 recruited onto the intron 1 region of MYCN. Knockdown of NCYM or OCT4 inhibited formation of spheres of neuroblastoma cells and promoted asymmetric cell division in MYCN-amplified human neuroblastoma cells. These results suggest that the functional interplay between MYCN, NCYM, and OCT4 contributes to aggressiveness of MYCN-amplified human neuroblastomas.
Keywords: MYCN; NCYM; OCT4; neuroblastoma; transcriptional regulation.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

