A randomised phase 1 study to investigate safety, pharmacokinetics and impact on gut microbiota following single and multiple oral doses in healthy male subjects of SMT19969, a novel agent for Clostridium difficile infections
- PMID: 25880933
- PMCID: PMC4349307
- DOI: 10.1186/s12879-015-0759-5
A randomised phase 1 study to investigate safety, pharmacokinetics and impact on gut microbiota following single and multiple oral doses in healthy male subjects of SMT19969, a novel agent for Clostridium difficile infections
Abstract
Background: Clostridium difficile infection (CDI) is a leading cause of diarrhoea in health care settings with symptoms ranging from mild and self-limiting to life threatening. SMT19969 is a novel, non-absorbable antibiotic currently under development for the treatment of CDI. Here we report the results from a Phase I study.
Methods: A double-blind, randomized, placebo-controlled study assessing safety and tolerability of single and multiple oral doses of SMT19969 in healthy volunteers. Pharmacokinetic assessments included blood and faecal sampling. The effect of food on systemic exposure and analysis of the gut microbiota were also included.
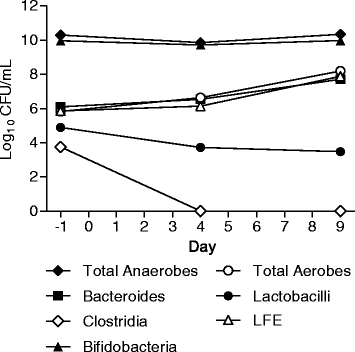
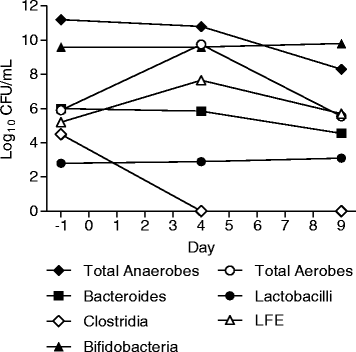
Results: Fifty-six healthy male subjects were enrolled. Following single oral doses of up to 2,000 mg in the fasted state, plasma concentrations of SMT19969 were generally below the lower limit of quantification. In the fed state levels ranged from 0.102 to 0.296 ng/mL after single dosing and after repeat dosing at Day 10 from 0.105 to 0.305 ng/mL. Following single and multiple oral doses of SMT19969, mean daily faecal concentrations increased with increasing dose level and were significantly above the typical MIC range for C. difficile (0.06-0.5 μg/mL). At 200 mg BID, mean (± SD) faecal concentrations of 1,466 (±547) μg/g and 1,364 (±446) μg/g were determined on days 5 and 10 of dosing respectively. No notable metabolites were detected in faeces. Overall, all doses of SMT19969 were well tolerated both as single oral doses or BID oral doses for 10 days. The majority (88%) of adverse events (AEs) were classified as gastrointestinal disorders and were mild in severity, resolving without treatment. The gut microbiota was analysed in the multiple dose groups with minimal changes observed in the bacterial groups analysed except for total clostridia which were reduced to below the limit of detection by day 4 of dosing.
Conclusions: Oral administration of SMT19969 was considered safe and well tolerated and was associated with negligible plasma concentrations after single and multiple doses. In addition, minimal disruption of normal gut microbiota was noted, confirming the highly selective spectrum of the compound. These results support the further clinical development of SMT19969 as an oral therapy for CDI.
Trial registration: Current Controlled Trials. ISRCTN10858225 .
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

