Exploring the transparency mechanism and evaluating the effect of public reporting on prescription: a protocol for a cluster randomized controlled trial
- PMID: 25881035
- PMCID: PMC4426648
- DOI: 10.1186/s12889-015-1454-6
Exploring the transparency mechanism and evaluating the effect of public reporting on prescription: a protocol for a cluster randomized controlled trial
Abstract
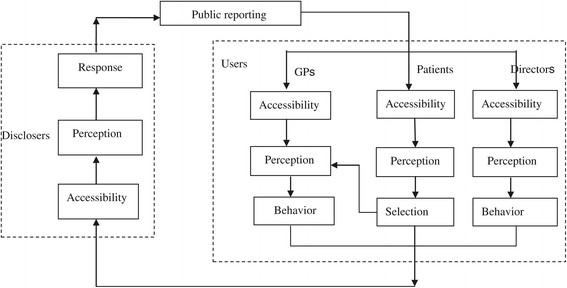
Background: The public reporting of health outcomes has become one of the most popular topics and is accepted as a quality improvement method in the healthcare field. However, little research has been conducted on the transparency mechanism, and results are mixed with regard to the evaluation of the effect of public reporting on quality improvement. The objectives of this trial are to investigate the transparency mechanism and to evaluate the effect of public reporting on prescription at the level of individual participants.
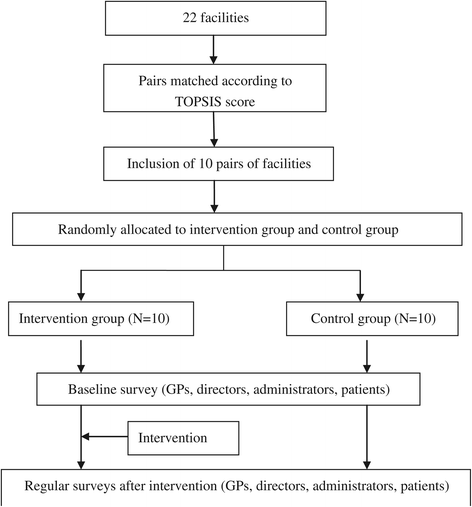
Methods/design: This study involves a cluster randomized controlled trial conducted in 20 primary-care facilities (clusters). Eligible clusters are those facilities with excellent hospital information systems and that have agreed to participate in the trial. The 20 clusters are matched into 10 pairs according to Technique for Order Preference by Similarity to Ideal Solution score. As the unit of randomization, each pair of facilities is assigned at random to a control or an intervention group through coin flipping. Prescribed ranking information is publicly reported in the intervention group. The public materials include the posters of individuals and of facilities, the ranking lists of general practitioners, and brochures of patients, which are updated monthly. The intervention began on 13th November 2013 and lasted for one year. Specifically, participants are surveyed at five points in time (baseline, quarterly following the intervention) through questionnaires, interviews, and observations. These participants include an average of 600 patients, 300 general practitioners, 15 directors, and 6 health bureau administrators. The primary outcomes are the transparency mechanism model and the changes in medicine-prescribe. Subsequently, the modifications in the transparency mechanism constructs are evaluated. The outcomes are measured at the individual participant level, and the professional who analyzes the data is blind to the randomization status.
Discussion: This study protocol outlines a design that aims to examine the transparency mechanism and to evaluate the effect of public reporting on prescription. The research design is significant in the field of public policy. Furthermore, this study intends to fill the gap of the investigation of the transparency mechanism and the evaluation of public reporting on prescription.
Figures
References
-
- HealthSparq. Health Care Transparency a Hot Topic at National Health Insurance Conference -- LAS VEGAS, July 16, 2013 PRNewswire-In.
-
- Hale TN. Transparency, accountability, and global governance. Glob Gov. 2008;14(1):73–94.
-
- Weil D, Fung A, Graham M, Fagotto E. The effectiveness of regulatory disclosure policies. J Policy Anal Manage. 2006;25(1):155–81. doi: 10.1002/pam.20160. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous