Are adaptive randomised trials or non-randomised studies the best way to address the Ebola outbreak in west Africa?
- PMID: 25881871
- PMCID: PMC7129402
- DOI: 10.1016/S1473-3099(15)70106-4
Are adaptive randomised trials or non-randomised studies the best way to address the Ebola outbreak in west Africa?
Abstract
The Ebola outbreak that has devastated parts of west Africa represents an unprecedented challenge for research and ethics. Estimates from the past three decades emphasise that the present effort to contain the epidemic in the three most affected countries (Guinea, Liberia, and Sierra Leone) has been insufficient, with more than 24,900 cases and about 10,300 deaths, as of March 25, 2015. Faced with such an exceptional event and the urgent response it demands, the use of randomised controlled trials (RCT) for Ebola-related research might be both unethical and infeasible and that potential interventions should be assessed in non-randomised studies on the basis of compassionate use. However, non-randomised studies might not yield valid conclusions, leading to large residual uncertainty about how to interpret the results, and can also waste scarce intervention-related resources, making them profoundly unethical. Scientifically sound and rigorous study designs, such as adaptive RCTs, could provide the best way to reduce the time needed to develop new interventions and to obtain valid results on their efficacy and safety while preserving the application of ethical precepts. We present an overview of clinical studies registered at present at the four main international trial registries and provide a simulation on how adaptive RCTs can behave in this context, when mortality varies simultaneously in either the control or the experimental group.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
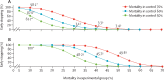
Comment in
-
Drug assessment in the Ebola virus disease epidemic in west Africa.Lancet Infect Dis. 2015 Nov;15(11):1258. doi: 10.1016/S1473-3099(15)00344-8. Lancet Infect Dis. 2015. PMID: 26531030 Free PMC article. No abstract available.
References
-
- Hooper C, Krishna S. This must be the year we beat Ebola in West Africa. https://theconversation.com/this-must-be-the-year-we-beat-ebola-in-west-... (accessed March 31, 2015).
-
- Brouqui P, Ippolito G. Ebola and travel—management of imported cases. Travel Med Infect Dis. 2014;12:561–562. - PubMed
-
- WHO Ebola Situation Report. 25 March 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-25-ma... (accessed March 30, 2015).
-
- WHO . Ethical considerations for use of unregistered interventions for Ebola viral disease: report of an advisory panel to WHO. World Health Organization Press; Geneva: Aug 11, 2014. http://www.who.int/csr/resources/publications/ebola/ethical-consideratio... (accessed Dec 1, 2014).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

