Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane
- PMID: 25882296
- PMCID: PMC4453867
- DOI: 10.1093/glycob/cwv021
Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane
Abstract
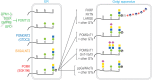
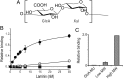
Associations between cells and the basement membrane are critical for a variety of biological events including cell proliferation, cell migration, cell differentiation and the maintenance of tissue integrity. Dystroglycan is a highly glycosylated basement membrane receptor, and is involved in physiological processes that maintain integrity of the skeletal muscle, as well as development and function of the central nervous system. Aberrant O-glycosylation of the α subunit of this protein, and a concomitant loss of dystroglycan's ability to function as a receptor for extracellular matrix (ECM) ligands that bear laminin globular (LG) domains, occurs in several congenital/limb-girdle muscular dystrophies (also referred to as dystroglycanopathies). Recent genetic studies revealed that mutations in DAG1 (which encodes dystroglycan) and at least 17 other genes disrupt the ECM receptor function of dystroglycan and cause disease. Here, we summarize recent advances in our understanding of the enzymatic functions of two of these disease genes: the like-glycosyltransferase (LARGE) and protein O-mannose kinase (POMK, previously referred to as SGK196). In addition, we discuss the structure of the glycan that directly binds the ECM ligands and the mechanisms by which this functional motif is linked to dystroglycan. In light of the fact that dystroglycan functions as a matrix receptor and the polysaccharide synthesized by LARGE is the binding motif for matrix proteins, we propose to name this novel polysaccharide structure matriglycan.
Keywords: LARGE; O-mannosyl glycan; POMK; dystroglycan; muscular dystrophy.
© The Author 2015. Published by Oxford University Press.
Figures

References
-
- Akhavan A, Crivelli SN, Singh M, Lingappa VR, Muschler JL. 2008. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 22:612–621. - PubMed
-
- Ashikov A, Buettner FF, Tiemann B, Gerardy-Schahn R, Bakker H. 2013. LARGE2 generates the same xylose- and glucuronic acid-containing glycan structures as LARGE. Glycobiology. 23:303–309. - PubMed
-
- Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F, et al. 2012. DPM2-CDG: A muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann Neurol. 72:550–558. - PubMed
-
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang WL, Schachter H, Dumanski JP, et al. 2004. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 10:696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

