As the fat flies: The dynamic lipid droplets of Drosophila embryos
- PMID: 25882628
- PMCID: PMC4516585
- DOI: 10.1016/j.bbalip.2015.04.002
As the fat flies: The dynamic lipid droplets of Drosophila embryos
Abstract
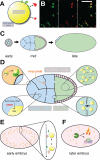
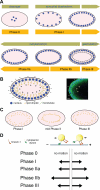
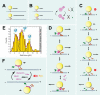
Research into lipid droplets is rapidly expanding, and new cellular and organismal roles for these lipid-storage organelles are continually being discovered. The early Drosophila embryo is particularly well suited for addressing certain questions in lipid-droplet biology and combines technical advantages with unique biological phenomena. This review summarizes key features of this experimental system and the techniques available to study it, in order to make it accessible to researchers outside this field. It then describes the two topics most heavily studied in this system, lipid-droplet motility and protein sequestration on droplets, discusses what is known about the molecular players involved, points to open questions, and compares the results from Drosophila embryo studies to what it is known about lipid droplets in other systems.
Keywords: Drosophila embryo; Lipid droplet; Microtubule motors; Protein sequestration.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

