Therapeutic hypothermia after nonshockable cardiac arrest: the HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial
- PMID: 25882712
- PMCID: PMC4353458
- DOI: 10.1186/s13049-015-0103-5
Therapeutic hypothermia after nonshockable cardiac arrest: the HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial
Abstract
Background: Meta-analyses of nonrandomized studies have provided conflicting data on therapeutic hypothermia, or targeted temperature management (TTM), at 33°C in patients successfully resuscitated after nonshockable cardiac arrest. Nevertheless, the latest recommendations issued by the International Liaison Committee on Resuscitation and by the European Resuscitation Council recommend therapeutic hypothermia. New data are available on the adverse effects of therapeutic hypothermia, notably infectious complications. The risk/benefit ratio of therapeutic hypothermia after nonshockable cardiac arrest is unclear.
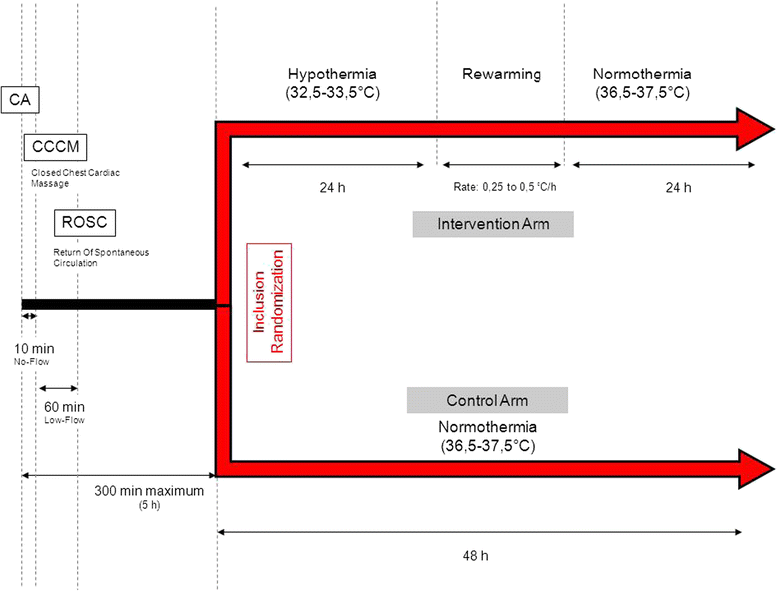
Methods: HYPERION is a multicenter (22 French ICUs) trial with blinded outcome assessment in which 584 patients with successfully resuscitated nonshockable cardiac arrest are allocated at random to either TTM between 32.5 and 33.5°C (therapeutic hypothermia) or TTM between 36.5 and 37.5°C (therapeutic normothermia) for 24 hours. Both groups are managed with therapeutic normothermia for the next 24 hours. TTM is achieved using locally available equipment. The primary outcome is day-90 neurological status assessed by the Cerebral Performance Categories (CPC) Scale with dichotomization of the results (1 + 2 versus 3 + 4 + 5). The primary outcome is assessed by a blinded psychologist during a semi-structured telephone interview of the patient or next of kin. Secondary outcomes are day-90 mortality, hospital mortality, severe adverse events, infections, and neurocognitive performance. The planned sample size of 584 patients will enable us to detect a 9% absolute difference in day-90 neurological status with 80% power, assuming a 14% event rate in the control group and a two-sided Type 1 error rate of 4.9%. Two interim analyses will be performed, after inclusion of 200 and 400 patients, respectively.
Discussion: The HYPERION trial is a multicenter, randomized, controlled, assessor-blinded, superiority trial that may provide an answer to an issue of everyday relevance, namely, whether TTM is beneficial in comatose patients resuscitated after nonshockable cardiac arrest. Furthermore, it will provide new data on the tolerance and adverse events (especially infectious complications) of TTM at 32.5-33.5°C.
Trial registration: ClinicalTrials.gov: NCT01994772 .
Figures

References
-
- Handley AJ, Koster R, Monsieurs K, Perkins GD, Davies S, Bossaert L. European Resuscitation Council Guidelines for Resuscitation 2005: Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2005;67:S7–23. doi: 10.1016/j.resuscitation.2005.10.007. - DOI - PubMed
-
- Rosomoff HL, Holaday DA. Cerebral Blood Flow and Cerebral Oxygen Consumption During Hypothermia. Am J Physiol. 1954;179:85–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

