The original sins of clinical trials with intravenous immunoglobulins in sepsis
- PMID: 25882822
- PMCID: PMC4343266
- DOI: 10.1186/s13054-015-0793-0
The original sins of clinical trials with intravenous immunoglobulins in sepsis
Abstract
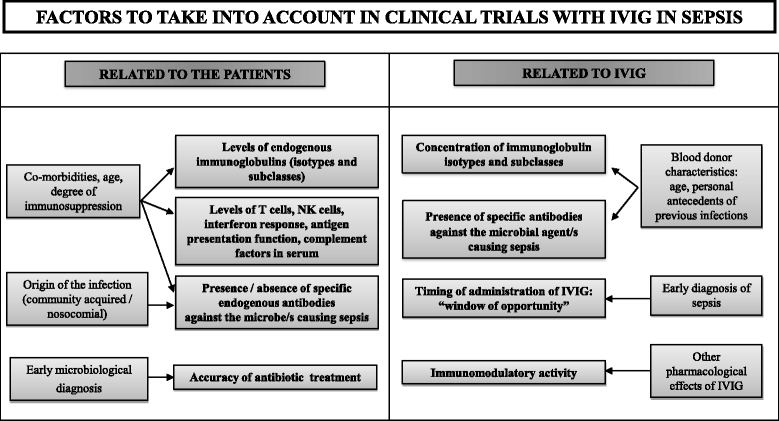
Intravenous immunoglobulins (IVIGs) have not yet demonstrated robust evidence in the benefit for treatment of sepsis. In spite of multiple clinical trials performed with IVIG in sepsis, it remains an experimental therapy for this severe condition. Nonetheless, these trials do not address a number of potential confounding factors, concerning both the patient and the IVIG preparations, which could greatly affect the final result. To name a few, endogenous levels of immunoglobulin isotypes and subclasses are not assessed prior to treatment. The presence/absence of patient antibodies against the microorganism(s) causing sepsis is not evaluated. The accuracy of antibiotic prescription is not included as an adjusting variable. The degree of patient immunosuppression (previous or induced by sepsis) is not documented. In turn, the concentration and antimicrobial specificities of the antibodies contained in the batches of IVIG are not assessed. Neither the pharmacokinetics of IVIG nor its potential immunomodulatory effects are evaluated. In addition, the concept of 'window of opportunity' for IVIG administration following diagnosis of sepsis is not considered. In conclusion, addressing these factors could help to individualise treatment with IVIG for sepsis, which could enhance the opportunities of this drug to show benefits in terms of survival in this severe condition.
Figures
References
-
- Päsler M, Dietz S, Werdan K. Hypogammaglobulinemia in Sepsis. In: Vincent PJ-L, editor. Annual Update in Intensive Care and Emergency Medicine 2012. Berlin Heidelberg: Springer; 2012. pp. 98–108.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources