Functional Effect of Pim1 Depends upon Intracellular Localization in Human Cardiac Progenitor Cells
- PMID: 25882843
- PMCID: PMC4447967
- DOI: 10.1074/jbc.M114.617431
Functional Effect of Pim1 Depends upon Intracellular Localization in Human Cardiac Progenitor Cells
Abstract
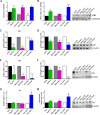
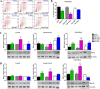
Human cardiac progenitor cells (hCPC) improve heart function after autologous transfer in heart failure patients. Regenerative potential of hCPCs is severely limited with age, requiring genetic modification to enhance therapeutic potential. A legacy of work from our laboratory with Pim1 kinase reveals effects on proliferation, survival, metabolism, and rejuvenation of hCPCs in vitro and in vivo. We demonstrate that subcellular targeting of Pim1 bolsters the distinct cardioprotective effects of this kinase in hCPCs to increase proliferation and survival, and antagonize cellular senescence. Adult hCPCs isolated from patients undergoing left ventricular assist device implantation were engineered to overexpress Pim1 throughout the cell (PimWT) or targeted to either mitochondrial (Mito-Pim1) or nuclear (Nuc-Pim1) compartments. Nuc-Pim1 enhances stem cell youthfulness associated with decreased senescence-associated β-galactosidase activity, preserved telomere length, reduced expression of p16 and p53, and up-regulation of nucleostemin relative to PimWT hCPCs. Alternately, Mito-Pim1 enhances survival by increasing expression of Bcl-2 and Bcl-XL and decreasing cell death after H2O2 treatment, thereby preserving mitochondrial integrity superior to PimWT. Mito-Pim1 increases the proliferation rate by up-regulation of cell cycle modulators Cyclin D, CDK4, and phospho-Rb. Optimal stem cell traits such as proliferation, survival, and increased youthful properties of aged hCPCs are enhanced after targeted Pim1 localization to mitochondrial or nuclear compartments. Targeted Pim1 overexpression in hCPCs allows for selection of the desired phenotypic properties to overcome patient variability and improve specific stem cell characteristics.
Keywords: Pim1; aging; apoptosis; heart failure; human cardiac progenitor cell; proliferation; senescence.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Ellison G. M., Torella D., Dellegrottaglie S., Perez-Martinez C., Perez de Prado A., Vicinanza C., Purushothaman S., Galuppo V., Iaconetti C., Waring C. D., Smith A., Torella M., Cuellas Ramon C., Gonzalo-Orden J. M., Agosti V., Indolfi C., Galiñanes M., Fernandez-Vazquez F., Nadal-Ginard B. (2011) Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll. Cardiol. 58, 977–986 - PubMed
-
- Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., Leri A., Kajstura J., Nadal-Ginard B., Anversa P. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 - PubMed
-
- Urbanek K., Torella D., Sheikh F., De Angelis A., Nurzynska D., Silvestri F., Beltrami C. A., Bussani R., Beltrami A. P., Quaini F., Bolli R., Leri A., Kajstura J., Anversa P. (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc. Natl. Acad. Sci. U.S.A. 102, 8692–8697 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL105759/HL/NHLBI NIH HHS/United States
- P01HL085577/HL/NHLBI NIH HHS/United States
- R01HL067245/HL/NHLBI NIH HHS/United States
- R01HL101217/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- R37HL091102/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- R01HL105759/HL/NHLBI NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
- R01HL117163/HL/NHLBI NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- R01 HL113656/HL/NHLBI NIH HHS/United States
- R01 HL117163/HL/NHLBI NIH HHS/United States
- R01HL087023/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

