Cranberry juice capsules and urinary tract infection after surgery: results of a randomized trial
- PMID: 25882919
- PMCID: PMC4519382
- DOI: 10.1016/j.ajog.2015.04.003
Cranberry juice capsules and urinary tract infection after surgery: results of a randomized trial
Abstract
Objective: The risk of urinary tract infection (UTI) among women undergoing elective gynecological surgery during which a catheter is placed is high: 10-64% following catheter removal. We conducted the first randomized, double-blind, placebo-controlled trial of the therapeutic efficacy of cranberry juice capsules in preventing UTI after surgery.
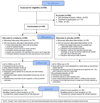
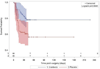
Study design: We recruited patients from a single hospital between August 2011 and January 2013. Eligible participants were undergoing elective gynecological surgery that did not involve a fistula repair or vaginal mesh removal. One hundred sixty patients were randomized and received 2 cranberry juice capsules 2 times a day, equivalent to 2 8 ounce servings of cranberry juice, for 6 weeks after surgery or matching placebo. The primary endpoint was the proportion of participants who experienced clinically diagnosed and treated UTI with or without positive urine culture. Kaplan-Meier plots and log rank tests compared the 2 treatment groups.
Results: The occurrence of UTI was significantly lower in the cranberry treatment group compared with the placebo group (15 of 80 [19%] vs 30 of 80 [38%]; odds ratio, 0.38; 95% confidence interval, 0.19-0.79; P = .008). After adjustment for known confounders, including the frequency of intermittent self-catheterization in the postoperative period, the protective effects of cranberry remained (odds ratio, 0.42; 95% confidence interval, 0.18-0.94). There were no treatment differences in the incidence of adverse events, including gastrointestinal upset (56% vs 61% for cranberry vs placebo).
Conclusion: Among women undergoing elective benign gynecological surgery involving urinary catheterization, the use of cranberry extract capsules during the postoperative period reduced the rate of UTI by half.
Keywords: catheter-associated urinary tract infection; clinical trial; cranberry extract.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Comment in
-
Cranberry capsules (2 taken twice daily for an average 38 days) reduce the risk of postoperative urinary tract infection in women undergoing benign gynaecological surgery involving intraoperative catheterisation.Evid Based Med. 2015 Aug;20(4):137. doi: 10.1136/ebmed-2015-110227. Epub 2015 Jun 30. Evid Based Med. 2015. PMID: 26126759 No abstract available.
-
Urine trouble without cranberries?Am J Obstet Gynecol. 2015 Aug;213(2):123-4. doi: 10.1016/j.ajog.2015.04.030. Am J Obstet Gynecol. 2015. PMID: 26216179 No abstract available.
References
-
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010 Mar 1;50(5):625–663. - PubMed
-
- Falagas ME, Athanasiou S, Iavazzo C, Tokas T, Antsaklis A. Urinary tract infections after pelvic floor gynecological surgery: prevalence and effect of antimicrobial prophylaxis. A systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 2008 Aug;19(8):1165–1172. - PubMed
-
- Foxman B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect Dis Clin North Am. 2014 Mar;28(1):1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

