In Vivo Role of INPP4B in Tumor and Metastasis Suppression through Regulation of PI3K-AKT Signaling at Endosomes
- PMID: 25883022
- PMCID: PMC4497843
- DOI: 10.1158/2159-8290.CD-14-1347
In Vivo Role of INPP4B in Tumor and Metastasis Suppression through Regulation of PI3K-AKT Signaling at Endosomes
Abstract
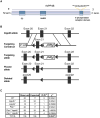
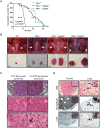
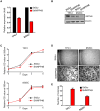
The phosphatases PTEN and INPP4B have been proposed to act as tumor suppressors by antagonizing PI3K-AKT signaling and are frequently dysregulated in human cancer. Although PTEN has been extensively studied, little is known about the underlying mechanisms by which INPP4B exerts its tumor-suppressive function and its role in tumorigenesis in vivo. Here, we show that a partial or complete loss of Inpp4b morphs benign thyroid adenoma lesions in Pten heterozygous mice into lethal and metastatic follicular-like thyroid cancer (FTC). Importantly, analyses of human thyroid cancer cell lines and specimens reveal INPP4B downregulation in FTC. Mechanistically, we find that INPP4B, but not PTEN, is enriched in the early endosomes of thyroid cancer cells, where it selectively inhibits AKT2 activation and in turn tumor proliferation and anchorage-independent growth. We therefore identify INPP4B as a novel tumor suppressor in FTC oncogenesis and metastasis through localized regulation of the PI3K-AKT pathway at the endosomes.
Significance: Although both PTEN and INPP4B can inhibit PI3K-AKT signaling through their lipid phosphatase activities, here we demonstrate lack of an epistatic relationship between the two tumor suppressors. Instead, the qualitative regulation of PI3K-AKT2 signaling by INPP4B provides a mechanism for their cooperation in suppressing thyroid tumorigenesis and metastasis.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


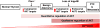
Comment in
-
INPP4B Is a Tumor Suppressor in the Context of PTEN Deficiency.Cancer Discov. 2015 Jul;5(7):697-700. doi: 10.1158/2159-8290.CD-15-0609. Cancer Discov. 2015. PMID: 26152921 Free PMC article.
References
-
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer research. 2003;63:196–206. - PubMed
-
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer research. 2004;64:3171–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

