Vesicles versus Tubes: Is Endoplasmic Reticulum-Golgi Transport in Plants Fundamentally Different from Other Eukaryotes?
- PMID: 25883241
- PMCID: PMC4453782
- DOI: 10.1104/pp.15.00124
Vesicles versus Tubes: Is Endoplasmic Reticulum-Golgi Transport in Plants Fundamentally Different from Other Eukaryotes?
Abstract
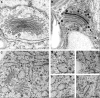
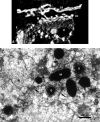
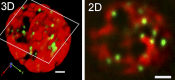
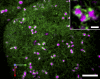
The endoplasmic reticulum (ER) is the gateway to the secretory pathway in all eukaryotic cells. Its products subsequently pass through the Golgi apparatus on the way to the cell surface (true secretion) or to the lytic compartment of the cell (vacuolar protein transport). In animal cells, the Golgi apparatus is present as a stationary larger order complex near the nucleus, and transport between the cortical ER and the Golgi complex occurs via an intermediate compartment which is transported on microtubules. By contrast, higher plant cells have discrete mobile Golgi stacks that move along the cortical ER, and the intermediate compartment is absent. Although many of the major molecular players involved in ER-Golgi trafficking in mammalian and yeast (Saccharomyces cerevisiae) cells have homologs in higher plants, the narrow interface (less than 500 nm) between the Golgi and the ER, together with the motility factor, makes the identification of the transport vectors responsible for bidirectional traffic between these two organelles much more difficult. Over the years, a controversy has arisen over the two major possibilities by which transfer can occur: through vesicles or direct tubular connections. In this article, four leading plant cell biologists attempted to resolve this issue. Unfortunately, their opinions are so divergent and often opposing that it was not possible to reach a consensus. Thus, we decided to let each tell his or her version individually. The review begins with an article by Federica Brandizzi that provides the necessary molecular background on coat protein complexes in relation to the so-called secretory units model for ER-Golgi transport in highly vacuolated plant cells. The second article, written by Chris Hawes, presents the evidence in favor of tubules. It is followed by an article from David Robinson defending the classical notion that transport occurs via vesicles. The last article, by Akihiko Nakano, introduces the reader to possible alternatives to vesicles or tubules, which are now emerging as a result of exciting new developments in high-resolution light microscopy in yeast.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures

References
-
- Andreeva AV, Kutuzov MA, Evans DE, Hawes CR (1998) The structure and function of the Golgi apparatus: a hundred years of questions. J Exp Bot 49: 1281–1291
-
- Appenzeller-Herzog C, Hauri HP (2006) The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci 119: 2173–2183 - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

