Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins
- PMID: 25883322
- PMCID: PMC4448682
- DOI: 10.1101/gr.184390.114
Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins
Abstract
RNA binding proteins of the conserved CUGBP1, Elav-like factor (CELF) family contribute to heart and skeletal muscle development and are implicated in myotonic dystrophy (DM). To understand their genome-wide functions, we analyzed the transcriptome dynamics following induction of CELF1 or CELF2 in adult mouse heart and of CELF1 in muscle by RNA-seq, complemented by crosslinking/immunoprecipitation-sequencing (CLIP-seq) analysis of mouse cells and tissues to distinguish direct from indirect regulatory targets. We identified hundreds of mRNAs bound in their 3' UTRs by both CELF1 and the developmentally induced MBNL1 protein, a threefold greater overlap in target messages than expected, including messages involved in development and cell differentiation. The extent of 3' UTR binding by CELF1 and MBNL1 predicted the degree of mRNA repression or stabilization, respectively, following CELF1 induction. However, CELF1's RNA binding specificity in vitro was not detectably altered by coincubation with recombinant MBNL1. These findings support a model in which CELF and MBNL proteins bind independently to mRNAs but functionally compete to specify down-regulation or localization/stabilization, respectively, of hundreds of mRNA targets. Expression of many alternative 3' UTR isoforms was altered following CELF1 induction, with 3' UTR binding associated with down-regulation of isoforms and genes. The splicing of hundreds of alternative exons was oppositely regulated by these proteins, confirming an additional layer of regulatory antagonism previously observed in a handful of cases. The regulatory relationships between CELFs and MBNLs in control of both mRNA abundance and splicing appear to have evolved to enhance developmental transitions in major classes of heart and muscle genes.
© 2015 Wang et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

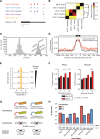
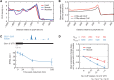

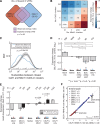
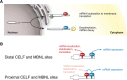
Similar articles
-
Mis-splicing of Tau exon 10 in myotonic dystrophy type 1 is reproduced by overexpression of CELF2 but not by MBNL1 silencing.Biochim Biophys Acta. 2011 Jul;1812(7):732-42. doi: 10.1016/j.bbadis.2011.03.010. Epub 2011 Mar 23. Biochim Biophys Acta. 2011. PMID: 21439371
-
Distribution of alternative untranslated regions within the mRNA of the CELF1 splicing factor affects its expression.Sci Rep. 2022 Jan 7;12(1):190. doi: 10.1038/s41598-021-03901-9. Sci Rep. 2022. PMID: 34996980 Free PMC article.
-
Identification of Targets of CUG-BP, Elav-Like Family Member 1 (CELF1) Regulation in Embryonic Heart Muscle.PLoS One. 2016 Feb 11;11(2):e0149061. doi: 10.1371/journal.pone.0149061. eCollection 2016. PLoS One. 2016. PMID: 26866591 Free PMC article.
-
The Muscleblind family of proteins: an emerging class of regulators of developmentally programmed alternative splicing.Differentiation. 2006 Mar;74(2-3):65-80. doi: 10.1111/j.1432-0436.2006.00060.x. Differentiation. 2006. PMID: 16533306 Review.
-
MicroRNA-Based Therapeutic Perspectives in Myotonic Dystrophy.Int J Mol Sci. 2019 Nov 9;20(22):5600. doi: 10.3390/ijms20225600. Int J Mol Sci. 2019. PMID: 31717488 Free PMC article. Review.
Cited by
-
Transcriptome-Wide Analysis Reveals Modulation of Human Macrophage Inflammatory Phenotype Through Alternative Splicing.Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1434-47. doi: 10.1161/ATVBAHA.116.307573. Epub 2016 May 26. Arterioscler Thromb Vasc Biol. 2016. PMID: 27230130 Free PMC article.
-
RNA Sequence Context Effects Measured In Vitro Predict In Vivo Protein Binding and Regulation.Mol Cell. 2016 Oct 20;64(2):294-306. doi: 10.1016/j.molcel.2016.08.035. Epub 2016 Oct 6. Mol Cell. 2016. PMID: 27720642 Free PMC article.
-
A Candidate RNAi Screen Reveals Diverse RNA-Binding Protein Phenotypes in Drosophila Flight Muscle.Cells. 2021 Sep 22;10(10):2505. doi: 10.3390/cells10102505. Cells. 2021. PMID: 34685485 Free PMC article.
-
Natural antisense transcripts in diseases: From modes of action to targeted therapies.Wiley Interdiscip Rev RNA. 2018 Mar;9(2):e1461. doi: 10.1002/wrna.1461. Epub 2018 Jan 17. Wiley Interdiscip Rev RNA. 2018. PMID: 29341438 Free PMC article. Review.
-
Extensive alternative splicing transitions during postnatal skeletal muscle development are required for calcium handling functions.Elife. 2017 Aug 11;6:e27192. doi: 10.7554/eLife.27192. Elife. 2017. PMID: 28826478 Free PMC article.
References
-
- Barreau C, Paillard L, Mereau A, Osborne HB. 2006. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie 88: 515–525. - PubMed
-
- Charlet BN, Logan P, Singh G, Cooper TA. 2002. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol Cell 9: 649–658. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- DP5 OD017865/OD/NIH HHS/United States
- T32 GM007287/GM/NIGMS NIH HHS/United States
- RC2HG005624/HG/NHGRI NIH HHS/United States
- R01HL045565/HL/NHLBI NIH HHS/United States
- R01 AR045653/AR/NIAMS NIH HHS/United States
- R01AR045653/AR/NIAMS NIH HHS/United States
- T32 GM087237/GM/NIGMS NIH HHS/United States
- R01 AR060733/AR/NIAMS NIH HHS/United States
- R01AR060733/AR/NIAMS NIH HHS/United States
- R01GM085319/GM/NIGMS NIH HHS/United States
- R01 HL045565/HL/NHLBI NIH HHS/United States
- OD017865-02/OD/NIH HHS/United States
- R01 GM085319/GM/NIGMS NIH HHS/United States
- RC2 HG005624/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
