Widespread exon skipping triggers degradation by nuclear RNA surveillance in fission yeast
- PMID: 25883323
- PMCID: PMC4448684
- DOI: 10.1101/gr.185371.114
Widespread exon skipping triggers degradation by nuclear RNA surveillance in fission yeast
Abstract
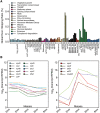
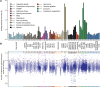
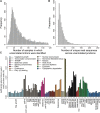
Exon skipping is considered a principal mechanism by which eukaryotic cells expand their transcriptome and proteome repertoires, creating different splice variants with distinct cellular functions. Here we analyze RNA-seq data from 116 transcriptomes in fission yeast (Schizosaccharomyces pombe), covering multiple physiological conditions as well as transcriptional and RNA processing mutants. We applied brute-force algorithms to detect all possible exon-skipping events, which were widespread but rare compared to normal splicing events. Exon-skipping events increased in cells deficient for the nuclear exosome or the 5'-3' exonuclease Dhp1, and also at late stages of meiotic differentiation when nuclear-exosome transcripts decreased. The pervasive exon-skipping transcripts were stochastic, did not increase in specific physiological conditions, and were mostly present at less than one copy per cell, even in the absence of nuclear RNA surveillance and during late meiosis. These exon-skipping transcripts are therefore unlikely to be functional and may reflect splicing errors that are actively removed by nuclear RNA surveillance. The average splicing rate by exon skipping was ∼ 0.24% in wild type and ∼ 1.75% in nuclear exonuclease mutants. We also detected approximately 250 circular RNAs derived from single or multiple exons. These circular RNAs were rare and stochastic, although a few became stabilized during quiescence and in splicing mutants. Using an exhaustive search algorithm, we also uncovered thousands of previously unknown splice sites, indicating pervasive splicing; yet most of these splicing variants were cryptic and increased in nuclear degradation mutants. This study highlights widespread but low frequency alternative or aberrant splicing events that are targeted by nuclear RNA surveillance.
© 2015 Bitton et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Alexander R, Beggs JD. 2010. Cross-talk in transcription, splicing and chromatin: who makes the first call? Biochem Soc Trans 38: 1251–1256. - PubMed
-
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. 2014. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56: 55–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
