Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection--the InC3 Study
- PMID: 25883387
- PMCID: PMC4601911
- DOI: 10.1093/infdis/jiv220
Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection--the InC3 Study
Abstract
Background: We aimed to characterize the natural history of hepatitis C virus (HCV) reinfection and spontaneous clearance following reinfection (reclearance), including predictors of HCV reclearance.
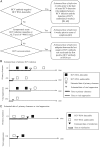
Methods: Data were synthesized from the 9 prospective cohorts of the International Collaboration of Incident Human Immunodeficiency Virus and HCV in Injecting Cohorts study, which evaluated HCV infection outcomes among people who inject drugs. Participants with primary HCV infection were classified as having achieved viral suppression if they had negative results of at least 1 subsequent HCV RNA test. Those with positive results of an HCV RNA test following viral suppression were investigated for reinfection. Viral sequence analysis was used to identify reinfection (defined as detection of heterologous virus with no subsequent detection of the original viral strain).
Results: Among 591 participants with acute primary HCV infection, 118 were investigated for reinfection. Twenty-eight participants were reinfected (12.3 cases/100 person-years; 95% confidence interval [CI], 8.5-17.8). Peak HCV RNA level was lower during reinfection than primary infection (P = .011). The proportion of individuals with reclearance 6 months after reinfection was 52% (95% CI, 33%-73%). After adjustment for study site, females with the IFNL4 (formerly IFNL3 and IL28B) rs12979860 CC genotype detected were more likely to have reclearance (hazard ratio, 4.16; 95% CI, 1.24-13.94; P = .021).
Conclusions: Sex and IFNL4 genotype are associated with spontaneous clearance after reinfection.
Keywords: IFNL4; cohort study; hepatitis C; injecting drug use; re-infection; sex; viral resolution.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006; 13:34–41. - PubMed
-
- Aitken CK, Lewis J, Tracy SL et al. . High incidence of hepatitis C virus reinfection in a cohort of injecting drug users. Hepatology 2008; 48:1746–52. - PubMed
-
- Micallef JM, Macdonald V, Jauncey M et al. . High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat 2007; 14:413–8. - PubMed
-
- van de Laar TJW, Molenkamp R, van den Berg C et al. . Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol 2009; 51:667–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

