Gene expression patterns and dynamics of the colonization of common bean (Phaseolus vulgaris L.) by highly virulent and weakly virulent strains of Fusarium oxysporum
- PMID: 25883592
- PMCID: PMC4383042
- DOI: 10.3389/fmicb.2015.00234
Gene expression patterns and dynamics of the colonization of common bean (Phaseolus vulgaris L.) by highly virulent and weakly virulent strains of Fusarium oxysporum
Abstract
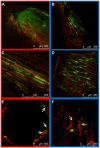
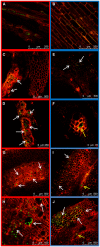
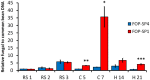
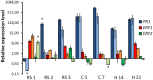
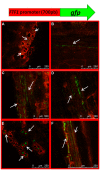
The dynamics of root and hypocotyl colonization, and the gene expression patterns of several fungal virulence factors and plant defense factors have been analyzed and compared in the interaction of two Fusarium oxysporum f. sp. phaseoli strains displaying clear differences in virulence, with a susceptible common bean cultivar. The growth of the two strains on the root surface and the colonization of the root was quantitatively similar although the highly virulent (HV) strain was more efficient reaching the central root cylinder. The main differences between both strains were found in the temporal and spatial dynamics of crown root and hypocotyl colonization. The increase of fungal biomass in the crown root was considerably larger for the HV strain, which, after an initial stage of global colonization of both the vascular cylinder and the parenchymal cells, restricted its growth to the newly differentiated xylem vessels. The weakly virulent (WV) strain was a much slower and less efficient colonizer of the xylem vessels, showing also growth in the intercellular spaces of the parenchyma. Most of the virulence genes analyzed showed similar expression patterns in both strains, except SIX1, SIX6 and the gene encoding the transcription factor FTF1, which were highly upregulated in root crown and hypocotyl. The response induced in the infected plant showed interesting differences for both strains. The WV strain induced an early and strong transcription of the PR1 gene, involved in SAR response, while the HV strain preferentially induced the early expression of the ethylene responsive factor ERF2.
Keywords: Fusarium oxysporum; Phaseolus vulgaris; confocal microscopy; effector; virulence.
Figures




References
-
- Alves-Santos F. A., Cordeiro-Rodrigues L., Sayagués J. M., Martín-Domínguez R., García-Benavides P., Crespo M. C., et al. (2002a). Pathogenicity and race characterization of Fusarium oxysporum f. sp. phaseoli isolates from Spain and Greece. Plant Pathol. 51 605–611 10.1046/j.1365-3059.2002.00745.x - DOI
-
- Armstrong G. M., Armstrong J. K. (1975). Reflections on the wilt fusaria. Annu. Rev. Phytopathol. 13 95–103 10.1146/annurev.py.13.090175.000523 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

