Campylobacter jejuni serine protease HtrA plays an important role in heat tolerance, oxygen resistance, host cell adhesion, invasion, and transmigration
- PMID: 25883795
- PMCID: PMC4397849
- DOI: 10.1556/EUJMI-D-15-00003
Campylobacter jejuni serine protease HtrA plays an important role in heat tolerance, oxygen resistance, host cell adhesion, invasion, and transmigration
Abstract
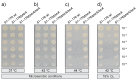
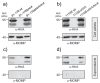
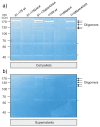
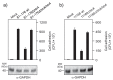
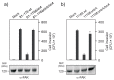
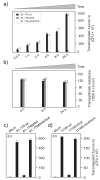
Campylobacter jejuni is an important pathogen of foodborne illness. Transmigration across the intestinal epithelial barrier and invasion are considered as primary reasons for tissue damage triggered by C. jejuni. Using knockout mutants, it was shown that the serine protease HtrA may be important for stress tolerance and physiology of C. jejuni. HtrA is also secreted in the extra-cellular environment, where it can cleave junctional host cell proteins such as E-cadherin. Aim of the present study was to establish a genetic complementation system in two C. jejuni strains in order to introduce the wild-type htrA gene in trans, test known htrA phenotypes, and provide the basis to perform further mutagenesis. We confirm that reexpression of the htrA wild-type gene in ΔhtrA mutants restored the following phenotypes: 1) C. jejuni growth at high temperature (44 °C), 2) growth under high oxygen stress conditions, 3) expression of proteolytically active HtrA oligomers, 4) secretion of HtrA into the supernatant, 5) cell attachment and invasion, and 6) transmigration across polarized epithelial cells. These results establish a genetic complementation system for htrA in C. jejuni, exclude polar effects in the ΔhtrA mutants, confirm important HtrA properties, and permit the discovery and dissection of new functions.
Keywords: E-cadherin; HtrA; cellular invasion; chaperone; flagellum; molecular pathogenesis; paracellular; secretion; signaling; stress response; transwell; virulence.
Conflict of interest statement
Figures

References
-
- World Health Organization. Foodborne Diseases. Geneva, Switzerland: WHO; http://www.who.int/foodsafety/areas_work/foodborne-diseases/en/; 2014.
-
- Young KT, Davis LM, DiRita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. - PubMed
-
- Nachamkin I, Szymanski CM, Blaser MJ. Campylobacter. Washington, DC: ASM Press; 2008.
-
- Oyarzabal OA, Backert S. Microbial Food Safety. New York: Springer; 2011.
-
- Talukder RK, Sutradhar SR, Rahman KM, Uddin MJ, Akhter H. Guillian–Barre syndrome. Mymensingh Med J. 2011;20:748–756. - PubMed
LinkOut - more resources
Full Text Sources
