Atypical centrioles during sexual reproduction
- PMID: 25883936
- PMCID: PMC4381714
- DOI: 10.3389/fcell.2015.00021
Atypical centrioles during sexual reproduction
Abstract
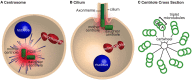
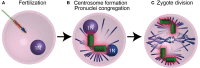
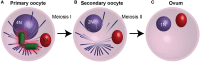
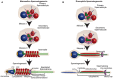
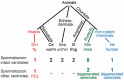
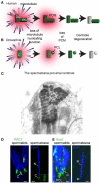
Centrioles are conserved, self-replicating, microtubule-based, 9-fold symmetric subcellular organelles that are essential for proper cell division and function. Most cells have two centrioles and maintaining this number of centrioles is important for animal development and physiology. However, how animals gain their first two centrioles during reproduction is only partially understood. It is well established that in most animals, the centrioles are contributed to the zygote by the sperm. However, in humans and many animals, the sperm centrioles are modified in their structure and protein composition, or they appear to be missing altogether. In these animals, the origin of the first centrioles is not clear. Here, we review various hypotheses on how centrioles are gained during reproduction and describe specialized functions of the zygotic centrioles. In particular, we discuss a new and atypical centriole found in sperm and zygote, called the proximal centriole-like structure (PCL). We also discuss another type of atypical centriole, the "zombie" centriole, which is degenerated but functional. Together, the presence of centrioles, PCL, and zombie centrioles suggests a universal mechanism of centriole inheritance among animals and new causes of infertility. Since the atypical centrioles of sperm and zygote share similar functions with typical centrioles in somatic cells, they can provide unmatched insight into centriole biology.
Keywords: centriole; centrosome; cilium; fertilization; microtubules; reproduction; sperm; zygote.
Figures



Similar articles
-
Atypical centrioles are present in Tribolium sperm.Open Biol. 2017 Mar;7(3):160334. doi: 10.1098/rsob.160334. Open Biol. 2017. PMID: 28298310 Free PMC article.
-
Centriole Remodeling during Spermiogenesis in Drosophila.Curr Biol. 2016 Dec 5;26(23):3183-3189. doi: 10.1016/j.cub.2016.07.006. Epub 2016 Oct 27. Curr Biol. 2016. PMID: 28094036 Free PMC article.
-
The origin of the second centriole in the zygote of Drosophila melanogaster.Genetics. 2014 May;197(1):199-205. doi: 10.1534/genetics.113.160523. Epub 2014 Feb 13. Genetics. 2014. PMID: 24532732 Free PMC article.
-
The Role of Sperm Centrioles in Human Reproduction - The Known and the Unknown.Front Cell Dev Biol. 2019 Oct 1;7:188. doi: 10.3389/fcell.2019.00188. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31632960 Free PMC article. Review.
-
It takes two (centrioles) to tango.Reproduction. 2019 Feb;157(2):R33-R51. doi: 10.1530/REP-18-0350. Reproduction. 2019. PMID: 30496124 Free PMC article. Review.
Cited by
-
Transition zone assembly and its contribution to axoneme formation in Drosophila male germ cells.J Cell Biol. 2016 Sep 26;214(7):875-89. doi: 10.1083/jcb.201603086. Epub 2016 Sep 19. J Cell Biol. 2016. PMID: 27646273 Free PMC article.
-
Human basal body basics.Cilia. 2016 Mar 14;5:13. doi: 10.1186/s13630-016-0030-8. eCollection 2016. Cilia. 2016. PMID: 26981235 Free PMC article. Review.
-
Dynamic Changes in the Transcriptome and Methylome of Chlamydomonas reinhardtii throughout Its Life Cycle.Plant Physiol. 2015 Dec;169(4):2730-43. doi: 10.1104/pp.15.00861. Epub 2015 Oct 8. Plant Physiol. 2015. PMID: 26450704 Free PMC article.
-
Conserved molecular interactions in centriole-to-centrosome conversion.Nat Cell Biol. 2016 Jan;18(1):87-99. doi: 10.1038/ncb3274. Epub 2015 Nov 23. Nat Cell Biol. 2016. PMID: 26595382 Free PMC article.
-
MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections.Int J Mol Sci. 2024 Jul 31;25(15):8358. doi: 10.3390/ijms25158358. Int J Mol Sci. 2024. PMID: 39125928 Free PMC article.
References
-
- Avidor-Reiss T., Gopalakrishnan J., Blachon S., Polyanovsky A. (2012). Centriole duplication and inheritance in Drosophila melanogaster, in The Centrosome: Cell and Molecular Mechanisms of Functions and Dysfunctions in Disease, ed Schatten H. (New York, NY: Humana Press; ), 3–31.
-
- Baccetti B., Afzelius B. A. (1976). The biology of the sperm cell. Monogr. Dev. Biol. 1–254. - PubMed
-
- Baccetti B., Burrini A. G., Dallai R., Pallini V. P., Periti P., Piantelli F., et al. . (1973). Structure and function in the spermatozoon of Bacillus rossius. The spermatozoon of arthropoda. XIX. J. Ultrastruct. Res. 12, 1–73. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

