Incomplete meiotic sex chromosome inactivation in the domestic dog
- PMID: 25884295
- PMCID: PMC4399420
- DOI: 10.1186/s12864-015-1501-9
Incomplete meiotic sex chromosome inactivation in the domestic dog
Abstract
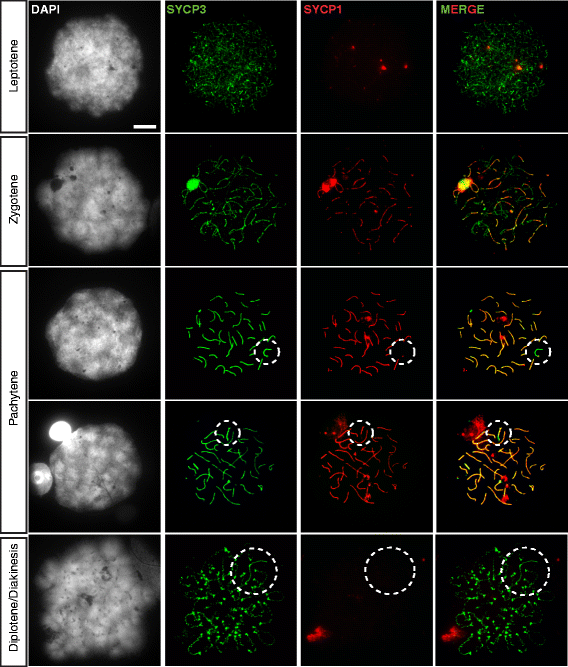
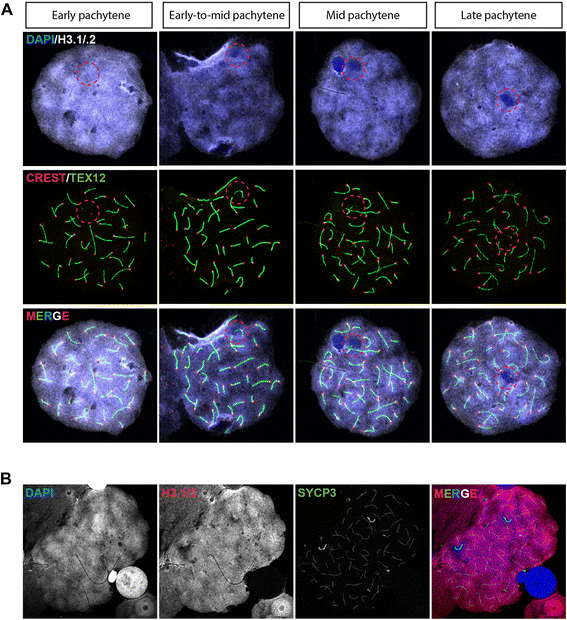
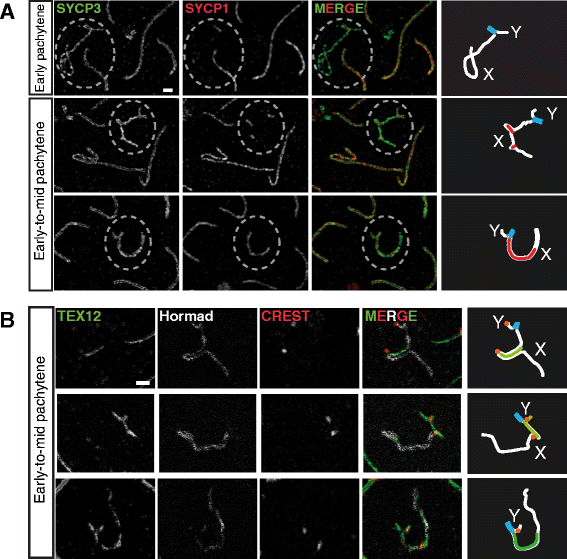
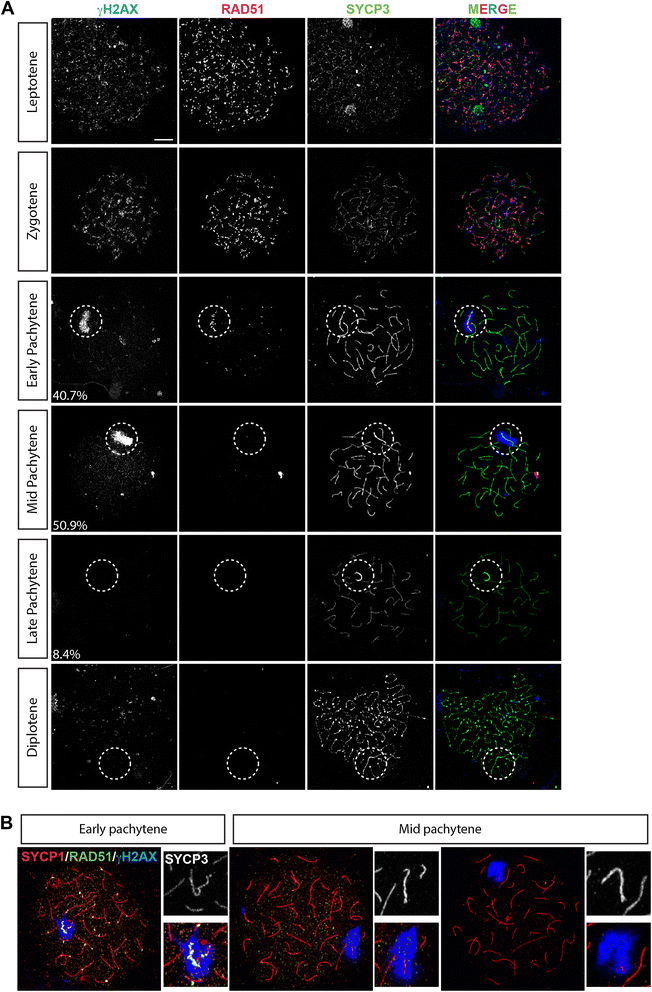
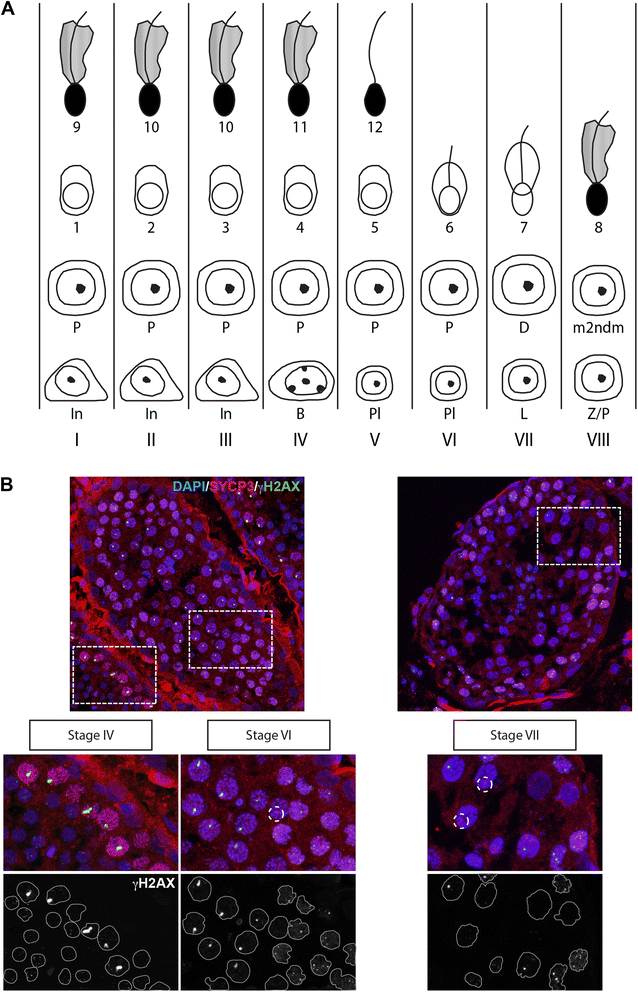
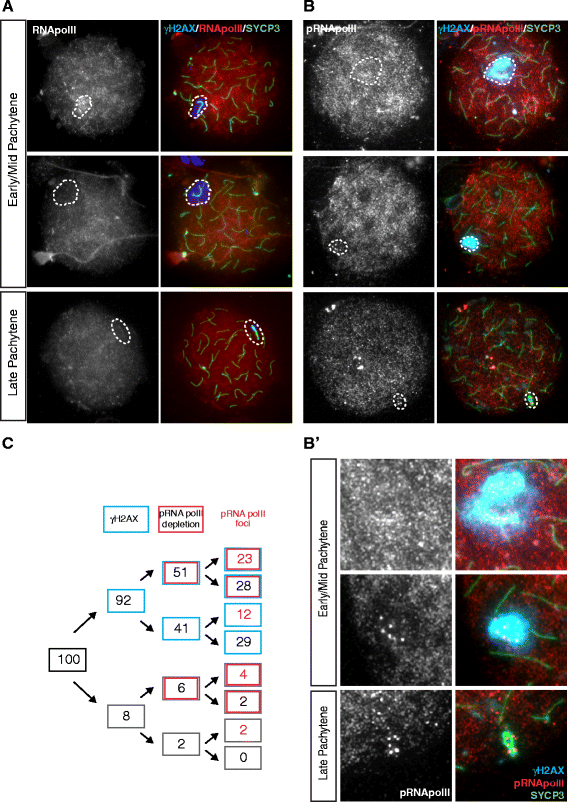
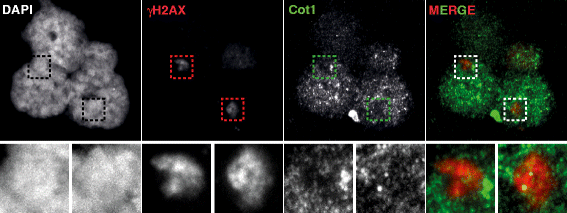
Background: In mammalian meiotic prophase, homologous chromosome recognition is aided by formation and repair of programmed DNA double-strand breaks (DSBs). Subsequently, stable associations form through homologous chromosome synapsis. In male mouse meiosis, the largely heterologous X and Y chromosomes synapse only in their short pseudoautosomal regions (PARs), and DSBs persist along the unsynapsed non-homologous arms of these sex chromosomes. Asynapsis of these arms and the persistent DSBs then trigger transcriptional silencing through meiotic sex chromosome inactivation (MSCI), resulting in formation of the XY body. This inactive state is partially maintained in post-meiotic haploid spermatids (postmeiotic sex chromatin repression, PSCR). For the human, establishment of MSCI and PSCR have also been reported, but X-linked gene silencing appears to be more variable compared to mouse. To gain more insight into the regulation and significance of MSCI and PSCR among different eutherian species, we have performed a global analysis of XY pairing dynamics, DSB repair, MSCI and PSCR in the domestic dog (Canis lupus familiaris), for which the complete genome sequence has recently become available, allowing a thorough comparative analyses.
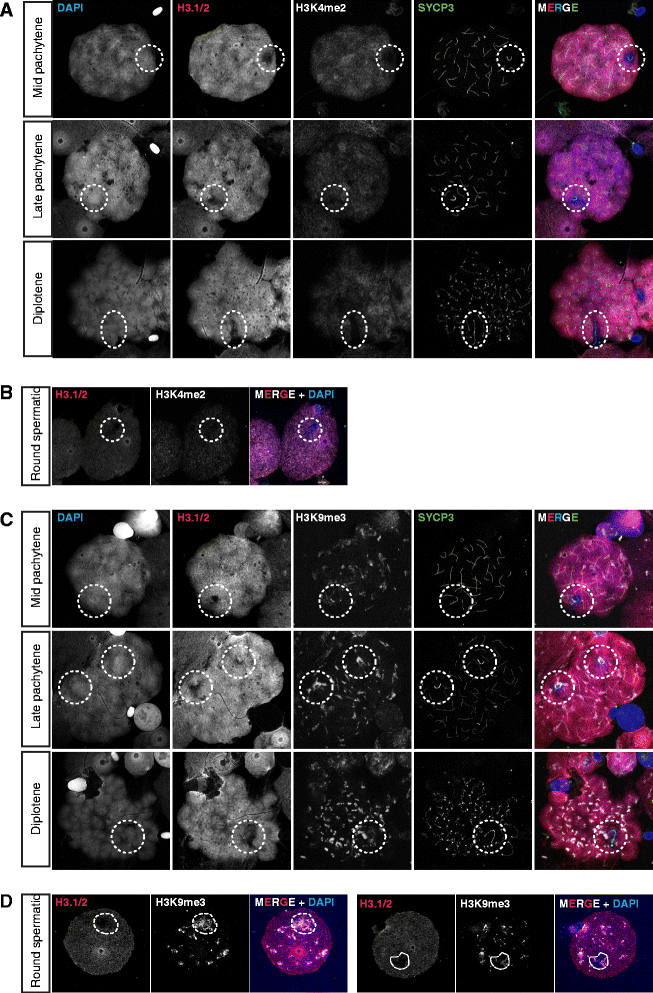
Results: In addition to PAR synapsis between X and Y, we observed extensive self-synapsis of part of the dog X chromosome, and rapid loss of known markers of DSB repair from that part of the X. Sequencing of RNA from purified spermatocytes and spermatids revealed establishment of MSCI. However, the self-synapsing region of the X displayed higher X-linked gene expression compared to the unsynapsed area in spermatocytes, and was post-meiotically reactivated in spermatids. In contrast, genes in the PAR, which are expected to escape MSCI, were expressed at very low levels in both spermatocytes and spermatids. Our comparative analysis was then used to identify two X-linked genes that may escape MSCI in spermatocytes, and 21 that are specifically re-activated in spermatids of human, mouse and dog.
Conclusions: Our data indicate that MSCI is incomplete in the dog. This may be partially explained by extensive, but transient, self-synapsis of the X chromosome, in association with rapid completion of meiotic DSB repair. In addition, our comparative analysis identifies novel candidate male fertility genes.
Figures
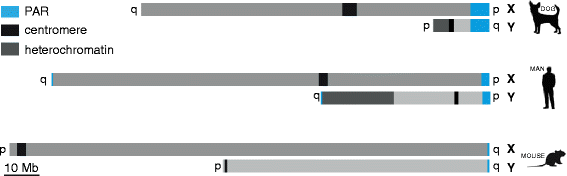


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

