Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial
- PMID: 25884460
- PMCID: PMC4377203
- DOI: 10.1186/s12884-015-0515-2
Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial
Abstract
Background: Venous thromboembolism (VTE) is a leading cause of maternal mortality and morbidity, with the highest incidence occurring during the postpartum period. This study compared the ability of two types of low-molecular-weight heparin, enoxaparin and bemiparin, to decrease the incidence of VTE following elective caesarean section, emergency caesarean section, and vaginal delivery in women who had risk factors for thromboembolism.
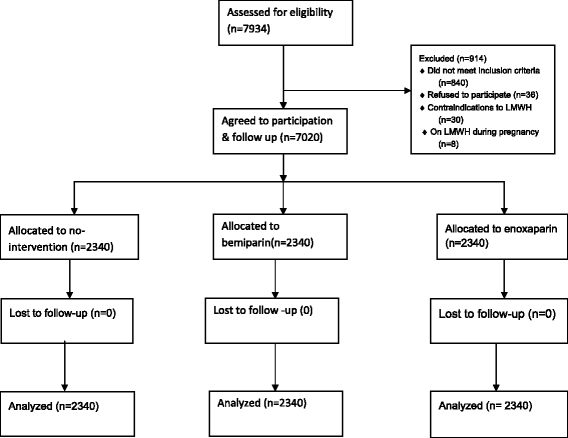
Methods: In this prospective clinical trial using a sequential group allocation method, 7020 haemodynamically stable women delivered vaginally or abdominally at the Maternity Teaching Hospital, Kurdistan region, Erbil, Iraq, between May 1, 2012, and November 1, 2013. These women had risk factors for VTE and were allocated to the following groups: treatment with 3500 IU/day of bemiparin, 4000 IU/day of enoxaparin, or no intervention (control). The first dose was administered 6 hours after vaginal or abdominal delivery, or 8 hours after delivery in women receiving spinal anaesthesia. Subsequent doses were administered daily for up to 6 days. The incidence of VTE was assessed for up to 40 days postpartum. Data were analyzed using the Statistical Package for Social Sciences version 19. Proportions were compared using the chi square test of association or Fisher's exact test. Binary logistic regression analysis was used with VTE as the dependent variable.
Results: VTE occurred in 1 (0.042%) woman in the bemiparin group, two (0.085%) women in the enoxaparin group, and nine (0.384%) women in the control group (P = 0.017). Regression analysis showed that women on bemiparin (OR = 0.106; 95% CI = 0.013-0.838) and enoxaparin (OR = 0.226; 95% CI = 0.049-1.049) were at lower risk of developing VTE than control women. Adverse events in the enoxaparin group included wound dehiscence, haematoma, and separation. None of these occurred in the bemiparin group.
Conclusions: Postpartum bemiparin is significantly effective as a prophylaxis for VTE. Wound complications develop after use of enoxaparin, but not after bemiparin.
Trial registration: ClinicalTrials.gov; Identifier: NCT01588171 ; date: April 26, 2012.
References
-
- Rosendaal FR. Risk factors for venous thrombotic disease. Thromb Haemost. 1999;82:610–9. - PubMed
-
- Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J, American College of Chest Physicians Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:844S–86. doi: 10.1378/chest.08-0761. - DOI - PubMed
-
- Bain E, Wilson A, Tooher R, Gates S, Davis LJ, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2014;2:CD001689. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous