Vedolizumab for the treatment of moderately to severely active ulcerative colitis
- PMID: 25884529
- PMCID: PMC6709861
- DOI: 10.1002/phar.1561
Vedolizumab for the treatment of moderately to severely active ulcerative colitis
Erratum in
-
Vedolizumab for the Treatment of Moderately to Severely Active Ulcerative Colitis.Pharmacotherapy. 2015 Oct;35(10):979. doi: 10.1002/phar.1651. Pharmacotherapy. 2015. PMID: 26497483 No abstract available.
Abstract
Ulcerative colitis is a chronic, idiopathic, inflammatory bowel disease characterized by a relapsing and remitting course. A substantial proportion of patients fail conventional therapies despite therapy with immunosuppressives and tumor necrosis factor antagonists. Accordingly, newer therapeutic agents that target disease-specific inflammation and minimize adverse events are required. Central to the pathogenesis of ulcerative colitis is an aberrant host response to commensal microorganisms with a resultant dysregulation of gut immune homeostasis and lymphocyte trafficking. Recently, a newer biologic, vedolizumab, which blocks lymphocyte trafficking, has been developed for use in moderate to severe ulcerative colitis. The efficacy of this agent has been demonstrated to be similar to that of other currently available biologics, and the selectivity of this agent in blocking lymphocyte migration to the gut has substantially reduced treatment-related adverse events. The drug has now been approved for use in the United States and Europe, and, although the exact positioning of this biologic in clinical practice is yet to be defined, it represents an important new chapter in our armamentarium of treatment options for this population. In this review, we will highlight key considerations to be made by providers when using this agent in clinical practice.
Keywords: inflammatory bowel disease; mucosal addressin cell adhesion molecule-1; ulcerative colitis; vedolizumab; α4β7.
© 2015 Pharmacotherapy Publications, Inc.
Conflict of interest statement
Figures

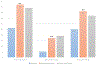
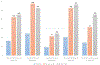

References
-
- Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. - PubMed
-
- Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther 2014;39:1095–103. - PubMed
-
- Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of Oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? evidence from cochrane reviews. Inflamm Bowel Dis 2013;19:2031–40. - PubMed
-
- Sherlock ME, Seow CH, Steinhart AH, et al. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2010:Cd007698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

