Enzyme architecture: optimization of transition state stabilization from a cation-phosphodianion pair
- PMID: 25884759
- PMCID: PMC4416717
- DOI: 10.1021/jacs.5b02202
Enzyme architecture: optimization of transition state stabilization from a cation-phosphodianion pair
Abstract
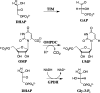
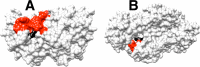
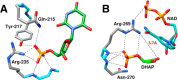
The side chain cation of R269 lies at the surface of l-glycerol 3-phosphate dehydrogenase (GPDH) and forms an ion pair to the phosphodianion of substrate dihydroxyacetone phosphate (DHAP), which is buried at the nonpolar protein interior. The R269A mutation of GPDH results in a 110-fold increase in K(m) (2.8 kcal/mol effect) and a 41,000-fold decrease in k(cat) (6.3 kcal/mol effect), which corresponds to a 9.1 kcal/mol destabilization of the transition state for GPDH-catalyzed reduction of DHAP by NADH. There is a 6.7 kcal/mol stabilization of the transition state for the R269A mutant GPDH-catalyzed reaction by 1.0 M guanidinium ion, and the transition state for the reaction of the substrate pieces is stabilized by an additional 2.4 kcal/mol by their covalent attachment at wildtype GPDH. These results provide strong support for the proposal that GPDH invests the 11 kcal/mol intrinsic phosphodianion binding energy of DHAP in trapping the substrate at a nonpolar active site, where strong electrostatic interactions are favored, and obtains a 9 kcal/mol return from stabilizing interactions between the side chain cation and transition state trianion. We propose a wide propagation for the catalytic motif examined in this work, which enables strong transition state stabilization from enzyme-phosphodianion pairs.
Figures



Similar articles
-
Enzyme Architecture: Self-Assembly of Enzyme and Substrate Pieces of Glycerol-3-Phosphate Dehydrogenase into a Robust Catalyst of Hydride Transfer.J Am Chem Soc. 2016 Nov 23;138(46):15251-15259. doi: 10.1021/jacs.6b09936. Epub 2016 Nov 10. J Am Chem Soc. 2016. PMID: 27792325 Free PMC article.
-
Human Glycerol 3-Phosphate Dehydrogenase: X-ray Crystal Structures That Guide the Interpretation of Mutagenesis Studies.Biochemistry. 2019 Feb 26;58(8):1061-1073. doi: 10.1021/acs.biochem.8b01103. Epub 2019 Jan 31. Biochemistry. 2019. PMID: 30640445 Free PMC article.
-
Enzyme Architecture: The Role of a Flexible Loop in Activation of Glycerol-3-phosphate Dehydrogenase for Catalysis of Hydride Transfer.Biochemistry. 2018 Jun 12;57(23):3227-3236. doi: 10.1021/acs.biochem.7b01282. Epub 2018 Feb 5. Biochemistry. 2018. PMID: 29337541 Free PMC article.
-
Specificity in transition state binding: the Pauling model revisited.Biochemistry. 2013 Mar 26;52(12):2021-35. doi: 10.1021/bi301491r. Epub 2013 Feb 4. Biochemistry. 2013. PMID: 23327224 Free PMC article. Review.
-
Enzyme activation through the utilization of intrinsic dianion binding energy.Protein Eng Des Sel. 2017 Mar 1;30(3):157-165. doi: 10.1093/protein/gzw064. Protein Eng Des Sel. 2017. PMID: 27903763 Free PMC article. Review.
Cited by
-
The role of ligand-gated conformational changes in enzyme catalysis.Biochem Soc Trans. 2019 Oct 31;47(5):1449-1460. doi: 10.1042/BST20190298. Biochem Soc Trans. 2019. PMID: 31657438 Free PMC article. Review.
-
Kinetics and mechanism for enzyme-catalyzed reactions of substrate pieces.Methods Enzymol. 2023;685:95-126. doi: 10.1016/bs.mie.2023.03.002. Epub 2023 Apr 18. Methods Enzymol. 2023. PMID: 37245916 Free PMC article.
-
Rate and Equilibrium Constants for an Enzyme Conformational Change during Catalysis by Orotidine 5'-Monophosphate Decarboxylase.Biochemistry. 2015 Jul 28;54(29):4555-64. doi: 10.1021/acs.biochem.5b00591. Epub 2015 Jul 14. Biochemistry. 2015. PMID: 26135041 Free PMC article.
-
Triosephosphate Isomerase: The Crippling Effect of the P168A/I172A Substitution at the Heart of an Enzyme Active Site.Biochemistry. 2023 Oct 17;62(20):2916-2927. doi: 10.1021/acs.biochem.3c00414. Epub 2023 Sep 28. Biochemistry. 2023. PMID: 37768194 Free PMC article.
-
Role of Loop-Clamping Side Chains in Catalysis by Triosephosphate Isomerase.J Am Chem Soc. 2015 Dec 9;137(48):15185-97. doi: 10.1021/jacs.5b09328. Epub 2015 Nov 30. J Am Chem Soc. 2015. PMID: 26570983 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

