The effect of individualized nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial protocol
- PMID: 25884881
- PMCID: PMC4352568
- DOI: 10.1186/s12885-015-1092-5
The effect of individualized nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial protocol
Abstract
Background: A low muscle mass is prevalent in patients with metastatic colorectal cancer (mCRC) and has been associated with poor treatment outcome. Chemotherapeutic treatment has an additional unfavorable effect on muscle mass. Sufficient protein intake and physical activity are known to induce muscle protein anabolism in healthy individuals, however it is unclear whether optimal nutrition is effective to preserve muscle mass in patients with mCRC during first-line chemotherapy as well. We hypothesize that individual nutritional counseling by a trained dietitian during first-line chemotherapy is effective in preserving muscle mass and may improve clinical outcomes in patients with mCRC.
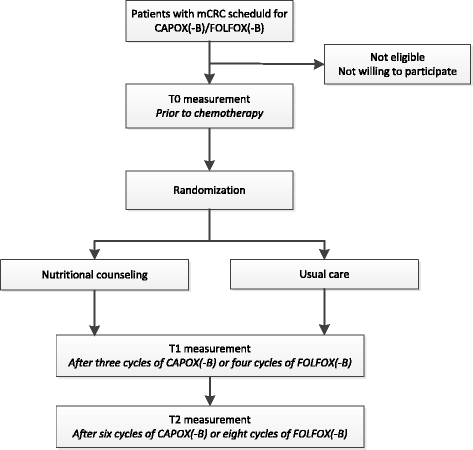
Methods/design: In this multi-center single-blind randomized controlled trial, patients with mCRC scheduled for first-line combination chemotherapy consisting of oxaliplatin and fluoropyrimidine, with or without bevacizumab (n = 110), will be randomized to receive either individualized nutritional counseling by a trained dietitian to achieve a sufficient dietary intake and an adequate physical activity level, or usual care. Outcome measures will be assessed at baseline and after two and four months of treatment. The primary endpoint will be the change in skeletal muscle area (measured by CT-scan) at the first treatment evaluation. Secondary endpoints will be quality of life, physical functioning, treatment toxicity, treatment intensity and survival. Statistical analyses include one-sided t-tests for the primary endpoint and mixed models and the Kaplan-Meier method for secondary endpoints.
Discussion: This randomized controlled trial will provide evidence whether individualized nutritional counseling during chemotherapy is effective in preventing loss of muscle mass in patients with mCRC.
Trial registration: ClinicalTrials.gov NCT01998152 ; Netherlands Trial Register NTR4223.
Figures
References
-
- GLOBOCAN 2012. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2014 [http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx]
-
- Peeters M, Siena S, Tabernero J, Douillard J, Koukakis R, Terwey J, et al. Survival outcomes in the PRIME study for patients (pts) with RAS/BRAF wild-type (WT) metastatic colorectal cancer (mCRC), by baseline Eastern Cooperative Oncology Group (ECOG) performance status (PS) [abstract] J Clin Oncol. 2014;32:5 s. doi: 10.1200/JCO.2013.49.4757. - DOI - PubMed
-
- Bazarbashi S, Aljubran AH, Al Zahrani A, Edesa W, Nabil-Ahmed M, Shoukri M: Early tumor shrinkage versus early response as a predictor for overall survival (OS) in patients with metastatic colorectal cancer (mCRC) treated with triplet chemotherapy regimens (TCR) [abstract]. Journal of Clinical Oncology 2014, 2014 (suppl; abstr e14580).
-
- Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. A randomized phase III trial of mFOLFOX6 plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment for metastatic colorectal cancer: West Japan Oncology Group study 4407G (WJOG4407G) [abstract] J Clin Oncol. 2014;32:5 s. doi: 10.1200/JCO.2013.49.4757. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical