Impact of diabetes type II and chronic inflammation on pancreatic cancer
- PMID: 25885700
- PMCID: PMC4336675
- DOI: 10.1186/s12885-015-1047-x
Impact of diabetes type II and chronic inflammation on pancreatic cancer
Abstract
Background: We explored if known risk factors for pancreatic cancer such as type II diabetes and chronic inflammation, influence the pathophysiology of an established primary tumor in the pancreas and if administration of metformin has an impact on tumor growth.
Methods: Pancreatic carcinomas were assessed in a syngeneic orthotopic pancreas adenocarcinoma model after injection of 6606PDA cells in the pancreas head of either B6.V-Lep(ob/ob) mice exhibiting a type II diabetes-like syndrome or normoglycemic mice. Chronic pancreatitis was then induced by repetitive administration of cerulein. Cell proliferation, cell death, inflammation and the expression of cancer stem cell markers within the carcinomas was evaluated by immunohistochemistry. In addition, the impact of the antidiabetic drug, metformin, on the pathophysiology of the tumor was assessed.
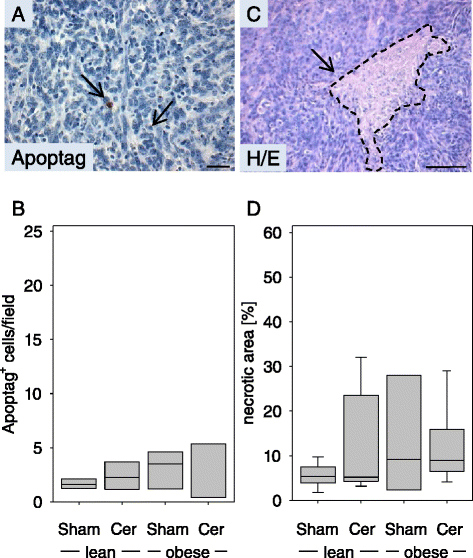
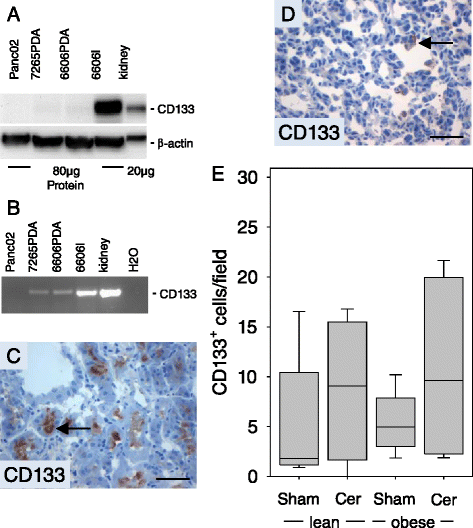
Results: Diabetic mice developed pancreatic ductal adenocarcinomas with significantly increased tumor weight when compared to normoglycemic littermates. Diabetes caused increased proliferation of cancer cells, but did not inhibit cancer cell necrosis or apoptosis. Diabetes also reduced the number of Aldh1 expressing cancer cells and moderately decreased the number of tumor infiltrating chloracetate esterase positive granulocytes. The administration of metformin reduced tumor weight as well as cancer cell proliferation. Chronic pancreatitis significantly diminished the pancreas weight and increased lipase activity in the blood, but only moderately increased tumor weight.
Conclusion: We conclude that diabetes type II has a fundamental influence on pancreatic ductal adenocarcinoma by stimulating cancer cell proliferation, while metformin inhibits cancer cell proliferation. Chronic inflammation had only a minor effect on the pathophysiology of an established adenocarcinoma.
Figures






References
-
- Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67(5):2187–96. doi: 10.1158/0008-5472.CAN-06-3281. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

