Active immunization with myelin-derived altered peptide ligand reduces mechanical pain hypersensitivity following peripheral nerve injury
- PMID: 25885812
- PMCID: PMC4340611
- DOI: 10.1186/s12974-015-0253-4
Active immunization with myelin-derived altered peptide ligand reduces mechanical pain hypersensitivity following peripheral nerve injury
Abstract
Background: T cells have been implicated in neuropathic pain that is caused by peripheral nerve injury. Immunogenic myelin basic protein (MBP) peptides have been shown to initiate mechanical allodynia in a T cell-dependent manner. Antagonistic altered peptide ligands (APLs) are peptides with substitutions in amino acid residues at T cell receptor contact sites and can inhibit T cell function and modulate inflammatory responses. In the present study, we studied the effects of immunization with MBP-derived APL on pain behavior and neuroinflammation in an animal model of peripheral nerve injury.
Methods: Lewis rats were immunized subcutaneously at the base of the tail with either a weakly encephalitogenic peptide of MBP (cyclo-MBP87-99) or APL (cyclo-(87-99)[A(91),A(96)]MBP87-99) in complete Freund's adjuvant (CFA) or CFA only (control), following chronic constriction injury (CCI) of the left sciatic nerve. Pain hypersensitivity was tested by measurements of paw withdrawal threshold to mechanical stimuli, regulatory T cells in spleen and lymph nodes were analyzed by flow cytometry, and immune cell infiltration into the nervous system was assessed by immunohistochemistry (days 10 and 30 post-CCI). Cytokines were measured in serum and nervous tissue of nerve-injured rats (day 10 post-CCI).
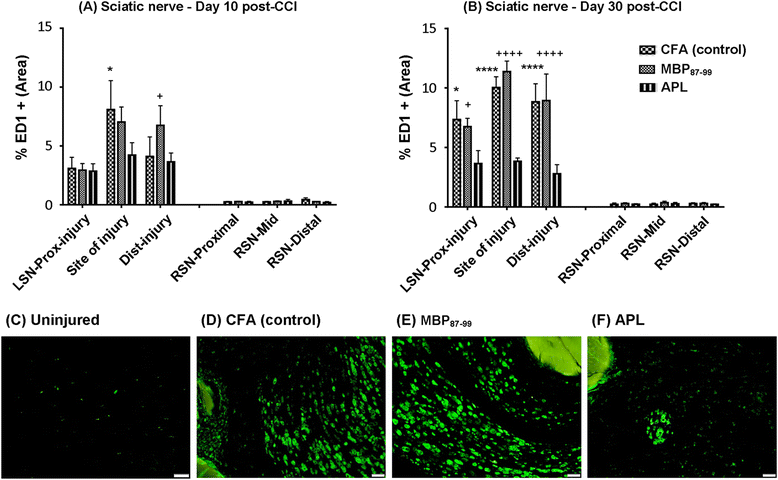
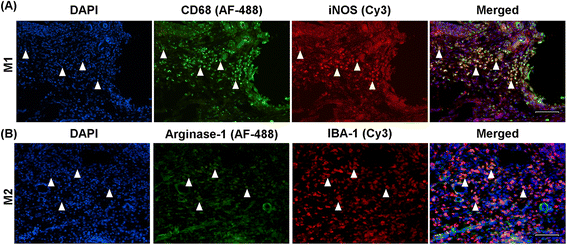
Results: Rats immunized with the APL cyclo-(87-99)[A(91),A(96)]MBP87-99 had significantly reduced mechanical pain hypersensitivity in the ipsilateral hindpaw compared to cyclo-MBP87-99-treated and control rats. This was associated with significantly decreased infiltration of T cells and ED1+ macrophages in the injured nerve of APL-treated animals. The percentage of anti-inflammatory (M2) macrophages was significantly upregulated in the APL-treated rats on day 30 post-CCI. Compared to the control rats, microglial activation in the ipsilateral lumbar spinal cord was significantly increased in the MBP-treated rats, but was not altered in the rats immunized with the MBP-derived APL. In addition, immunization with the APL significantly increased splenic regulatory T cells. Several cytokines were significantly altered after CCI, but no significant difference was observed between the APL-treated and control rats.
Conclusions: These results suggest that immune deviation by active immunization with a non-encephalitogenic MBP-derived APL mediates an analgesic effect in animals with peripheral nerve injury. Thus, T cell immunomodulation warrants further investigation as a possible therapeutic strategy for the treatment of peripheral neuropathic pain.
Figures








Similar articles
-
Effects of active immunisation with myelin basic protein and myelin-derived altered peptide ligand on pain hypersensitivity and neuroinflammation.J Neuroimmunol. 2015 Sep 15;286:59-70. doi: 10.1016/j.jneuroim.2015.07.004. Epub 2015 Jul 13. J Neuroimmunol. 2015. PMID: 26298325
-
Chronic Sciatic Neuropathy in Rat Reduces Voluntary Wheel-Running Activity With Concurrent Chronic Mechanical Allodynia.Anesth Analg. 2017 Jan;124(1):346-355. doi: 10.1213/ANE.0000000000001662. Anesth Analg. 2017. PMID: 27782944 Free PMC article.
-
The prolonged analgesic effect of epidural ropivacaine in a rat model of neuropathic pain.Anesth Analg. 2008 Jan;106(1):313-20, table of contents. doi: 10.1213/01.ane.0000296460.91012.51. Anesth Analg. 2008. PMID: 18165597
-
T cell-based therapeutic vaccination for spinal cord injury.Prog Brain Res. 2002;137:401-6. doi: 10.1016/s0079-6123(02)37031-6. Prog Brain Res. 2002. PMID: 12440382 Review.
-
Active and Passive Immunization with Myelin Basic Protein as a Method for Early Treatment of Traumatic Spinal Cord Injury; a Meta-Analysis.Arch Acad Emerg Med. 2021 Aug 30;9(1):e57. doi: 10.22037/aaem.v9i1.1316. eCollection 2021. Arch Acad Emerg Med. 2021. PMID: 34580655 Free PMC article. Review.
Cited by
-
Reduced GABAergic neuronal activity in zona incerta causes neuropathic pain in a rat sciatic nerve chronic constriction injury model.J Pain Res. 2017 May 11;10:1125-1134. doi: 10.2147/JPR.S131104. eCollection 2017. J Pain Res. 2017. PMID: 28546770 Free PMC article.
-
A myelin basic protein fragment induces sexually dimorphic transcriptome signatures of neuropathic pain in mice.J Biol Chem. 2020 Jul 31;295(31):10807-10821. doi: 10.1074/jbc.RA120.013696. Epub 2020 Jun 12. J Biol Chem. 2020. PMID: 32532796 Free PMC article.
-
Structural homology of myelin basic protein and muscarinic acetylcholine receptor: Significance in the pathogenesis of complex regional pain syndrome.Mol Pain. 2018 Jan-Dec;14:1744806918815005. doi: 10.1177/1744806918815005. Epub 2018 Nov 5. Mol Pain. 2018. PMID: 30392459 Free PMC article. Review.
-
Pain kept under wraps of myelin sheath.Front Pain Res (Lausanne). 2025 Apr 23;6:1569515. doi: 10.3389/fpain.2025.1569515. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40337526 Free PMC article.
-
On Peptides and Altered Peptide Ligands: From Origin, Mode of Action and Design to Clinical Application (Immunotherapy).Int Arch Allergy Immunol. 2016;170(4):211-233. doi: 10.1159/000448756. Epub 2016 Sep 20. Int Arch Allergy Immunol. 2016. PMID: 27642756 Free PMC article. Review.
References
-
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. - DOI - PubMed
-
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–22. doi: 10.1523/JNEUROSCI.4569-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

