Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study
- PMID: 25886002
- PMCID: PMC4428002
- DOI: 10.1186/s12885-015-1275-0
Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study
Abstract
Background: In large epidemiological studies it is often challenging to obtain biological samples. Self-sampling by study participants using dried blood spots (DBS) technique has been suggested to overcome this challenge. DBS is a type of biosampling where blood samples are obtained by a finger-prick lancet, blotted and dried on filter paper. However, the feasibility and efficacy of collecting DBS samples from study participants in large-scale epidemiological studies is not known. The aim of the present study was to test the feasibility and response rate of collecting self-sampled DBS and saliva samples in a population-based study of women above 50 years of age.
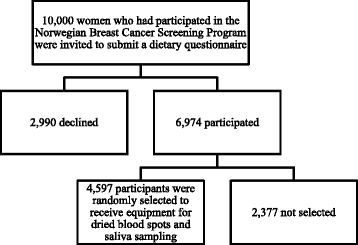
Methods: We determined response proportions, number of phone calls to the study center with questions about sampling, and quality of the DBS. We recruited women through a study conducted within the Norwegian Breast Cancer Screening Program. Invitations, instructions and materials were sent to 4,597 women. The data collection took place over a 3 month period in the spring of 2009.
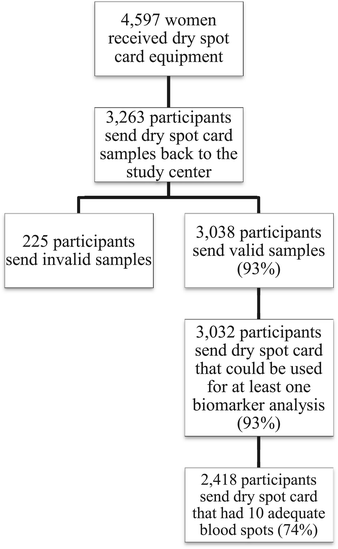
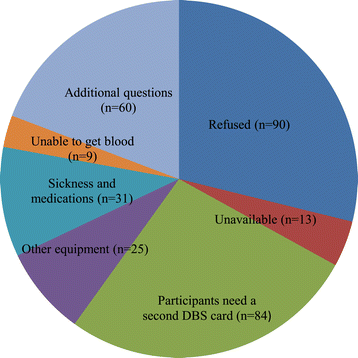
Results: Response proportions for the collection of DBS and saliva samples were 71.0% (3,263) and 70.9% (3,258), respectively. We received 312 phone calls (7% of the 4,597 women) with questions regarding sampling. Of the 3,263 individuals that returned DBS cards, 3,038 (93.1%) had been packaged and shipped according to instructions. A total of 3,032 DBS samples were sufficient for at least one biomarker analysis (i.e. 92.9% of DBS samples received by the laboratory). 2,418 (74.1%) of the DBS cards received by the laboratory were filled with blood according to the instructions (i.e. 10 completely filled spots with up to 7 punches per spot for up to 70 separate analyses). To assess the quality of the samples, we selected and measured two biomarkers (carotenoids and vitamin D). The biomarker levels were consistent with previous reports.
Conclusion: Collecting self-sampled DBS and saliva samples through the postal services provides a low cost, effective and feasible alternative in epidemiological studies.
Figures
References
-
- Pomelova VG, Osin NS. Prospects of the integration of dry blood spot technology with human health and environmental population studies. Vestn Ross Akad Med Nauk. 2007;12:10–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources