Phenotypic and molecular insights into CASK-related disorders in males
- PMID: 25886057
- PMCID: PMC4449965
- DOI: 10.1186/s13023-015-0256-3
Phenotypic and molecular insights into CASK-related disorders in males
Abstract
Background: Heterozygous loss-of-function mutations in the X-linked CASK gene cause progressive microcephaly with pontine and cerebellar hypoplasia (MICPCH) and severe intellectual disability (ID) in females. Different CASK mutations have also been reported in males. The associated phenotypes range from nonsyndromic ID to Ohtahara syndrome with cerebellar hypoplasia. However, the phenotypic spectrum in males has not been systematically evaluated to date.
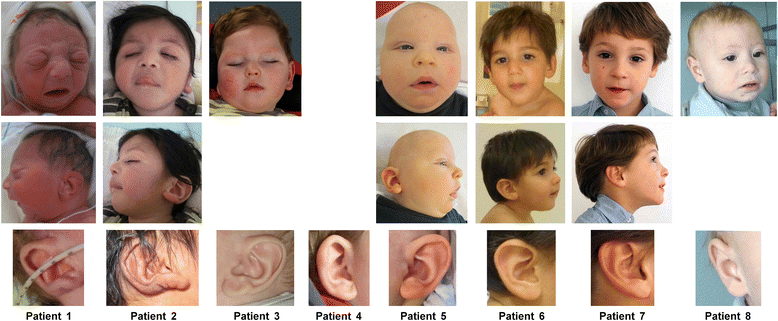
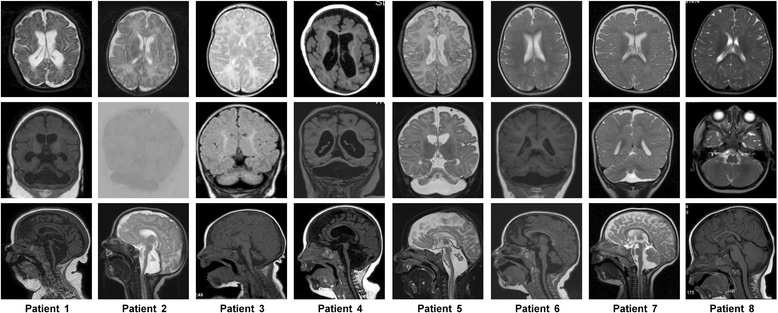
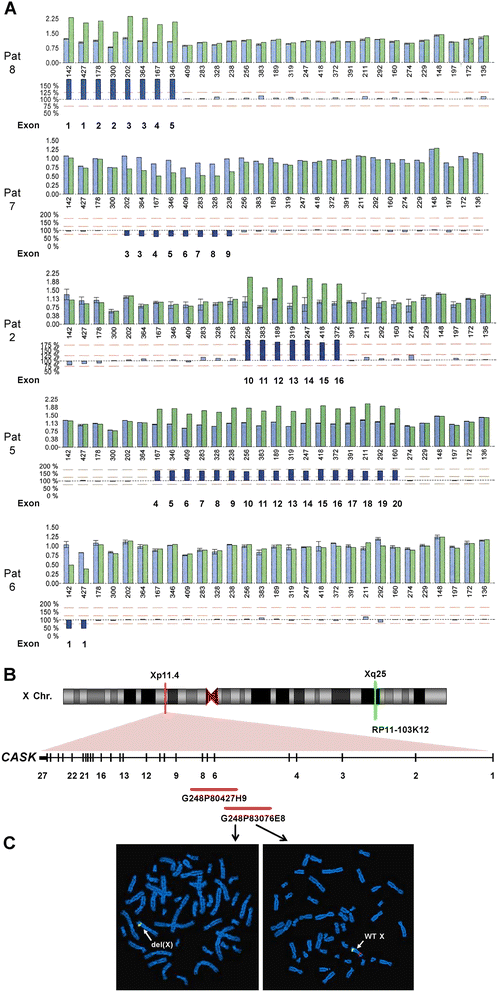
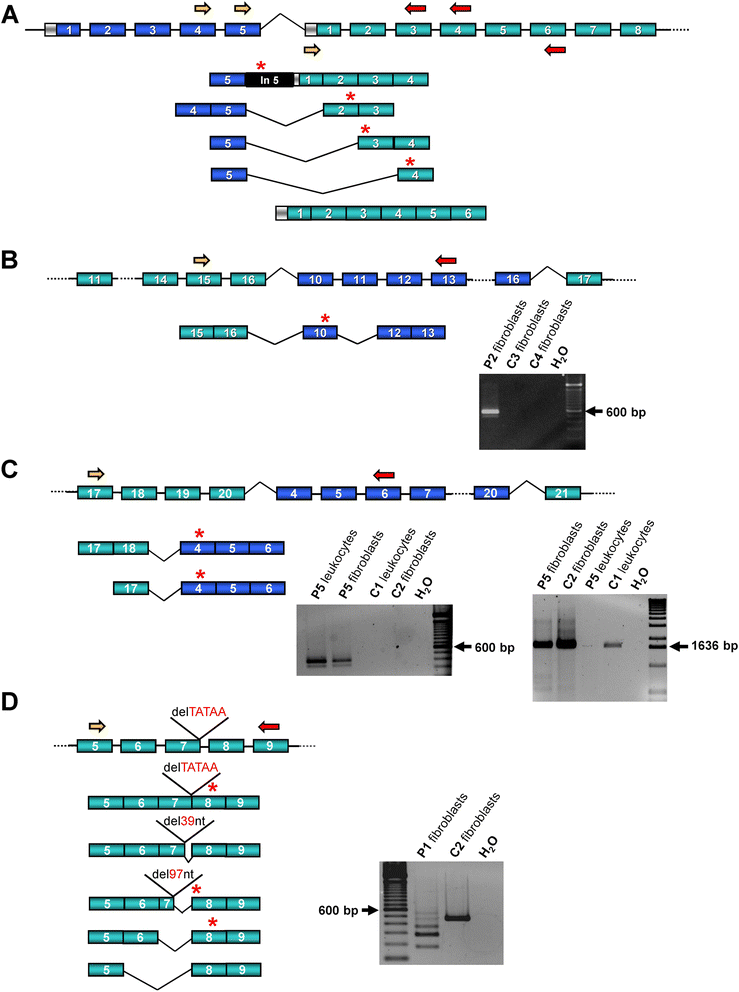
Methods: We identified a CASK alteration in 8 novel unrelated male patients by targeted Sanger sequencing, copy number analysis (MLPA and/or FISH) and array CGH. CASK transcripts were investigated by RT-PCR followed by sequencing. Immunoblotting was used to detect CASK protein in patient-derived cells. The clinical phenotype and natural history of the 8 patients and 28 CASK-mutation positive males reported previously were reviewed and correlated with available molecular data.
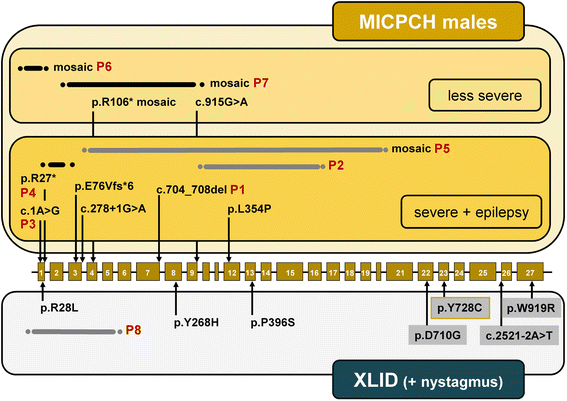
Results: CASK alterations include one nonsense mutation, one 5-bp deletion, one mutation of the start codon, and five partial gene deletions and duplications; seven were de novo, including three somatic mosaicisms, and one was familial. In three subjects, specific mRNA junction fragments indicated in tandem duplication of CASK exons disrupting the integrity of the gene. The 5-bp deletion resulted in multiple aberrant CASK mRNAs. In fibroblasts from patients with a CASK loss-of-function mutation, no CASK protein could be detected. Individuals who are mosaic for a severe CASK mutation or carry a hypomorphic mutation still showed detectable amount of protein.
Conclusions: Based on eight novel patients and all CASK-mutation positive males reported previously three phenotypic groups can be distinguished that represent a clinical continuum: (i) MICPCH with severe epileptic encephalopathy caused by hemizygous loss-of-function mutations, (ii) MICPCH associated with inactivating alterations in the mosaic state or a partly penetrant mutation, and (iii) syndromic/nonsyndromic mild to severe ID with or without nystagmus caused by CASK missense and splice mutations that leave the CASK protein intact but likely alter its function or reduce the amount of normal protein. Our findings facilitate focused testing of the CASK gene and interpreting sequence variants identified by next-generation sequencing in cases with a phenotype resembling either of the three groups.
Figures

References
-
- Burglen L, Chantot-Bastaraud S, Garel C, Milh M, Touraine R, Zanni G, et al. Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet J Rare Dis. 2012;7:18. doi: 10.1186/1750-1172-7-18. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

