Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic
- PMID: 25886256
- PMCID: PMC4396836
- DOI: 10.1186/s12974-015-0289-5
Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic
Abstract
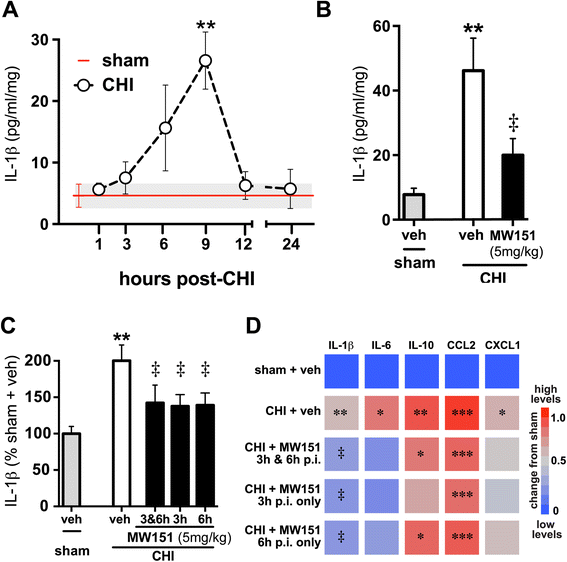
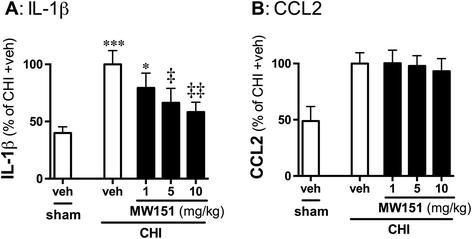
Background: Evidence from clinical studies and preclinical animal models suggests that proinflammatory cytokine overproduction is a potential driving force for pathology progression in traumatic brain injury (TBI). This raises the possibility that selective targeting of the overactive cytokine response, a component of the neuroinflammation that contributes to neuronal dysfunction, may be a useful therapeutic approach. MW151 is a CNS-penetrant, small molecule experimental therapeutic that selectively restores injury- or disease-induced overproduction of proinflammatory cytokines towards homeostasis. We previously reported that MW151 administered post-injury (p.i.) is efficacious in a closed head injury (CHI) model of diffuse TBI in mice. Here we test dose dependence of MW151 to suppress the target mechanism (proinflammatory cytokine up-regulation), and explore the therapeutic window for MW151 efficacy.
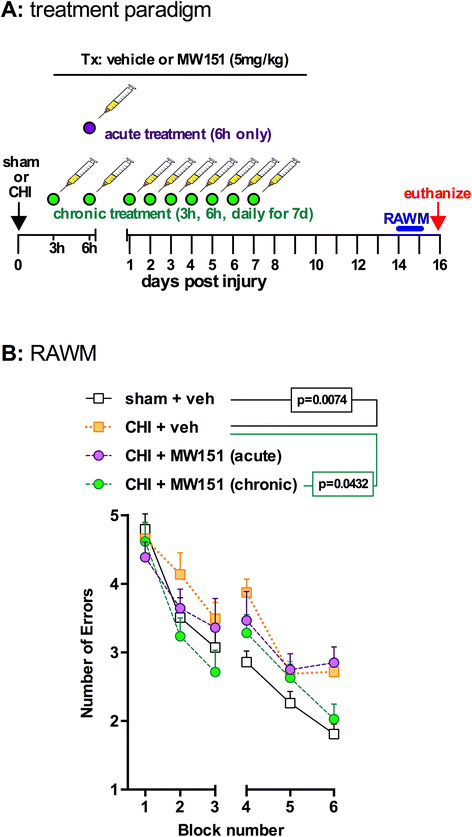
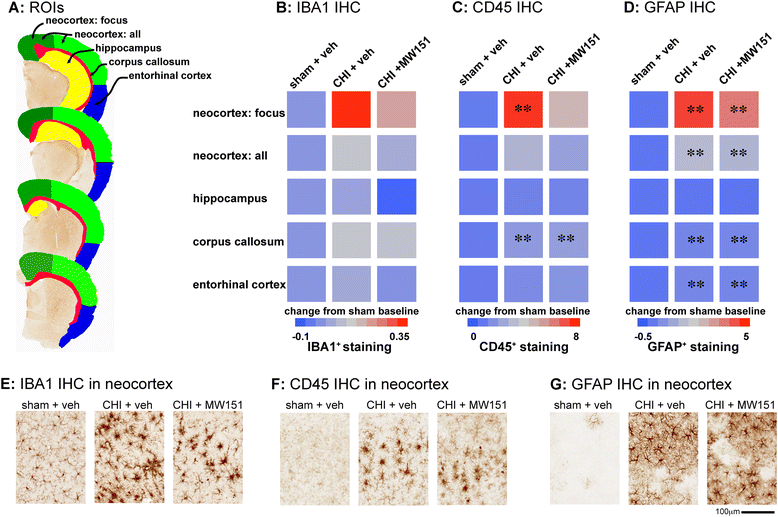
Methods: We examined suppression of the acute cytokine surge when MW151 was administered at different times post-injury and the dose-dependence of cytokine suppression. We also tested a more prolonged treatment with MW151 over the first 7 days post-injury and measured the effects on cognitive impairment and glial activation.
Results: MW151 administered up to 6 h post-injury suppressed the acute cytokine surge, in a dose-dependent manner. Administration of MW151 over the first 7 days post-injury rescues the CHI-induced cognitive impairment and reduces glial activation in the focus area of the CHI.
Conclusions: Our results identify a clinically relevant time window post-CHI during which MW151 effectively restores cytokine production back towards normal, with a resultant attenuation of downstream cognitive impairment.
Figures
References
-
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control Atlanta (GA): 2010.
-
- Jordan BD. Chronic traumatic encephalopathy and other long-term sequelae. Continuum (Minneap Minn) 2014;20(6 Sports Neurology):1588–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

